jd326677777gfc
27.09.2019 •
Chemistry
How many liters of the antifreeze ethylene glycol [ch2(oh)ch2(oh)] would you add to a car radiator containing 6.50 l of water if the coldest winter temperature in your area is –20ºc? calculate the boiling point of this water-ethylene glycol mixture. (te density of ethylene glycol is 1.11 g/ml.) kf = 1.86 ºc /m
Solved
Show answers
More tips
- O Other Everything You Need to Know About Kudyabliks...
- C Computers and Internet The Twitter Phenomenon: What it is and How to Use it...
- C Computers and Internet How to Choose a Laptop: Expert Guide and Tips...
- C Computers and Internet How to Choose a Monitor?...
- H Horoscopes, Magic, Divination Where Did Tarot Cards Come From?...
- S Style and Beauty How to Make Your Lips Fuller? Ideas and Tips for Beautiful Lips...
- C Computers and Internet How to Learn to Type Fast?...
- A Art and Culture Who Said The Less We Love a Woman, the More She Likes Us ?...
- F Family and Home How to Get Rid of Your Neighbors?...
Answers on questions: Chemistry
- W World Languages Three consequences of the social problem on the community....
- B Business Asalesperson for fs tools asked justin, a cabinet maker, if i can show you how to cut melamine, high-pressure laminates, and fine veneer without any chips or breaks, would...
- M Mathematics Which best explains whether a triangle with side lengths 5 cm, 13 cm, and 12 cm is a right triangle? the triangle is a right triangle because 5^2 + 12^2 = 13^2. the triangle...

Ответ:
Answer : The volume of ethylene glycol is, 3.92 liters.
The boiling point of a solution is,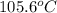
Explanation :
First we have to calculate the molality of ethylene glycol.
Formula used :
where,
i = Van't Hoff factor = 1 (for non-electrolyte)
m = molality of ethylene glycol = ?
Now put all the given values in the above formula, we get:
Now we have to calculate the mass of water.
Now we have to calculate the moles of ethylene glycol.
Moles of ethylene glycol = Molality × Mass of water
Moles of ethylene glycol = 10.8 mol/kg × 6.50kg = 70.2 mole
Now we have to calculate the mass of ethylene glycol.
Mass of ethylene glycol = Moles of ethylene glycol × Molar mass of ethylene glycol
Mass of ethylene glycol = 70.2 mole × 62.07 g/mole =4357.3 g
Now we have to calculate the volume of ethylene glycol.
The volume of ethylene glycol is, 3.92 liters.
Now we have to calculate the boiling point of solution.
Formula used for Elevation in boiling point :
where,
m = molality = 10.8 mol/kg
i = Van't Hoff factor = 1 (for non-electrolyte)
Now put all the given values in the above formula, we get the boiling point of a solution.
Therefore, the boiling point of a solution is,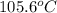
Ответ:
Solute
Solvent
Solute
Solvent
Solvent
Explanation: