zanedog2018
18.07.2019 •
Chemistry
Hydrazine, n2h4 , reacts with oxygen to form nitrogen gas and water. n2h4(aq)+o2(g)⟶n2(g)+2h2o(l) if 2.65 g of n2h4 reacts with excess oxygen and produces 0.350 l of n2 , at 295 k and 1.00 atm, what is the percent yield of the reaction?
Solved
Show answers
More tips
- A Animals and plants What Do Terriers Eat?...
- F Food and Cooking Discover the Benefits and Properties of Dates...
- C Computers and Internet Dynamically Assigned IP Address: What Is It and How Does It Work?...
- S Style and Beauty How to Get Rid of Acne: Scientifically Proven Methods...
- H Health and Medicine Simple Ways to Lower Cholesterol in the Blood: Tips and Tricks...
- O Other How to Choose the Best Answer to Your Question on The Grand Question ?...
- L Leisure and Entertainment History of International Women s Day: When Did the Celebration of March 8th Begin?...
- S Style and Beauty Intimate Haircut: The Reasons, Popularity, and Risks...
- A Art and Culture When Will Eurovision 2011 Take Place?...
- S Style and Beauty How to Choose the Perfect Hair Straightener?...
Answers on questions: Chemistry
- C Chemistry If you were balancing an equation containing the O2 molecule, which of the following would be correct representations of O2 and its coefficient? a. ½O2 b. O2 c. 3O2 d. 6O2...
- B Biology What do plants produce during photosynthesis? F oxygen G light H magnetic energy J carbon dioxide...
- S Social Studies Which kind of federalism saw the state and national governments working together? a. creative b. dual c. new d. cooperative...
- M Mathematics Question 3 2 pts Alex said that the values of x and y are the same. He further said that to find these unknown values, he would divide 26 by 3. Is he right? Justify your answer....

Ответ:
The percent yield of the reaction is 17.41 %.
Explanation:
ForTo calculate the number of moles, we use the equation:
Given mass of = 2.65 g
= 2.65 g
Molar mass of = 32.04 g/mol
= 32.04 g/mol
Putting values in above equation, we get:

ForTo calculate the number of moles, we use the equation given by ideal gas equation:
where,
P = pressure of the gas = 1 atm
V = Volume of gas = 0.350 L
n = Number of moles = ?
R = Gas constant =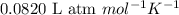
T = temperature of the gas = 273 K
Putting values in above equation, we get:
Now, to calculate the experimental yield of , we use the equation:
, we use the equation:
We are given:
Moles of nitrogen gas = 0.0144 moles
Molar mass of nitrogen gas = 28 g/mol
Putting values in above equation, we get:
Experimental yield of nitrogen gas = 0.4032 g
For the given chemical equation:By Stoichiometry of the reaction:
1 mole of produces 1 mole of nitrogen gas.
produces 1 mole of nitrogen gas.
So, 0.0827 moles of will produce =
will produce =  of nitrogen gas.
of nitrogen gas.
Now, to calculate the theoretical yield of nitrogen gas, we use equation 1:
Moles of nitrogen gas = 0.0827 mol
Molar mass nitrogen gas = 28 g/mol
Putting values in above equation, we get:
Theoretical yield of nitrogen gas = 2.3156 g
To calculate the percentage yield of nitrogen gas, we use the equation:Experimental yield of nitrogen gas = 0.4032 g
Theoretical yield of nitrogen gas = 2.3156 g
Putting values in above equation, we get:
Hence, the percent yield of the reaction is 17.41 %.
Ответ: