Hydrocyanic acid, hcn, is a weak acid. (a) write the chemical equation for the dissociation of hcn in water. (b) identify the brønsted-lowry conjugate acid-base pairs in the equation above. (c) write the chemical equation for the reaction of hcn with naoh. (d) write the chemical equation for dissociation of nacn in water. (e) write the chemical equation for the reaction of nacn and hci.
Solved
Show answers
More tips
- A Animals and plants What Do Terriers Eat?...
- F Food and Cooking Discover the Benefits and Properties of Dates...
- C Computers and Internet Dynamically Assigned IP Address: What Is It and How Does It Work?...
- S Style and Beauty How to Get Rid of Acne: Scientifically Proven Methods...
- H Health and Medicine Simple Ways to Lower Cholesterol in the Blood: Tips and Tricks...
- O Other How to Choose the Best Answer to Your Question on The Grand Question ?...
- L Leisure and Entertainment History of International Women s Day: When Did the Celebration of March 8th Begin?...
- S Style and Beauty Intimate Haircut: The Reasons, Popularity, and Risks...
- A Art and Culture When Will Eurovision 2011 Take Place?...
- S Style and Beauty How to Choose the Perfect Hair Straightener?...
Answers on questions: Chemistry
- C Chemistry This is an example of a(n) A. Aquatic Biome B. Terrestrial Biome C. Aquatic Biosphere D. Terrestrial Biosphere...
- C Chemistry How are isotopes used to determine average atomic mass?....
- C Chemistry Carbon 12, 13, and 14 are isotopes of the same element. They share the same place on the periodic table but possess varying numbers of neutrons. If carbon 12 has 6 protons, how many...
- C Chemistry Point b is between points a and c. Ab = 2x â 3, bc = 7x â 10, and ac = 32. What is the length of bc?....
- C Chemistry Which of these af3 molecules will have a nonzero dipole moment?....
- C Chemistry Randy observed that cooked onions in his food taste very differently from the raw onions that he had on his salad. he wondered why that was so. he thinks that he has an idea, but...
- C Chemistry Unlike the cell wall, the cell membrane is found in all cells?...
- C Chemistry What is the density of a substance with a mass of 45.00 g and a volume of 26.5ml?...
- C Chemistry Is light given off in small particles called photons...
- C Chemistry If an object is moving at a constant velocity and you want to change its momentum you would change its...

Ответ:
a)
b)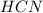 : acid
: acid  :conjugate base.
:conjugate base.
And, : base
: base  : conjugate acid.
: conjugate acid.
c)
d)
e)
Explanation:
a) Weak acid is defined as the acid which does not completely dissociates when dissolved in water. They have high pH. These releases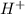 ions in their aqueous states.
ions in their aqueous states.
The equation for the dissociation of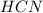 acid is given by:
acid is given by:
b) According to the Bronsted-Lowry conjugate acid-base theory, an acid is defined as a substance which looses donates protons and thus forming conjugate base and a base is defined as a substance which accepts protons and thus forming conjugate acid.
For the given chemical equation:
And, is gaining a proton, thus it is considered as a base and after gaining a proton, it forms
is gaining a proton, thus it is considered as a base and after gaining a proton, it forms  which is a conjugate acid.
which is a conjugate acid.
c) Neutralization reaction is a reaction in which an acid reacts with base to produce salt and water.
d) The chemical equation for dissociation of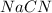 in water.
in water.
e) The chemical equation for the reaction of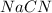 and
and 
Ответ: