amykookie24
01.03.2021 •
Chemistry
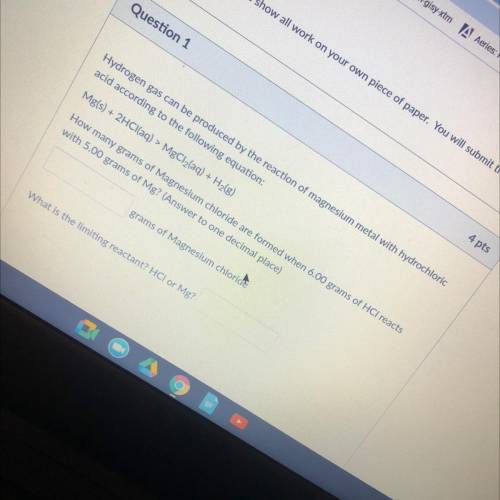
Hydrogen gas can be produced by the reaction of magnesium metal with hydrochloric
acid according to the following equation:
Mg(s) + 2HCl(aq) > MgCl2(aq) + H2(g)
How many grams of Magnesium chloride are formed when 6.00 grams of HCl reacts
with 5.00 grams of Mg? (Answer to one decimal place)
grams of Magnesium chloride
What is the limiting reactant? HCI or Mg?
Solved
Show answers
More tips
- P Philosophy How did the concept of module arise in computer science?...
- F Food and Cooking How to Cook Julienne? Recipes and Tips...
- H Health and Medicine At What Age Does a Person Stop Growing?...
- F Family and Home How to Choose a Name for Your Baby?...
- F Food and Cooking Discover the Health Benefits of Cedar Nuts...
- L Leisure and Entertainment Scrapbooking: What is it and Why is it Becoming More Popular?...
- L Leisure and Entertainment Carving: History and Techniques for Creating Vegetable and Fruit Decorations...
- F Food and Cooking How to Make Sushi: A Step-by-Step Guide to Perfectly Rolled Delights...
- T Travel and tourism Maldives Adventures: What is the Best Season to Visit the Luxurious Beaches?...
- F Food and Cooking Аэрогриль: Все, что нужно знать для подготовки здоровой и вкусной пищи...
Answers on questions: Chemistry
- C Chemistry The speed at the first quarter checkpoint is The speed at the second quarter checkpoint is m/s. The speed at the third quarter checkpoint is m/s. The speed at the finish...
- C Chemistry 13 A 4.85 g sample of anhydrous sodium sulfate is dissolved in water and the solution made up to250 cm in a volumetric flask.What is the concentration in mol dm- of sodium...
- C Chemistry Why do you think there are many hydrocarbon compound...
- C Chemistry PLEASE ANSWER ASSSAP A container with four different gases has a total pressure of 5.5 atmospheres. If Gas W has a partial pressure of 1.3 atmospheres, Gas X has a partial...
- C Chemistry Which procedure was carried out in the fume hood because it produced a toxic gas? A. copper(II) sulfate was added to ammonium hydroxide B. copper metal was added to sodium...
- C Chemistry What are the best techniques to remove the insoluble binder/filler materials from hcl - treated calcilum enriched tablet?...
- C Chemistry Rosa uses a microscope to look at a group of cells. She sees that the cells are joined together, so she knows that they are from one organism. She also sees that all of...
- C Chemistry The way a student prepares for a test or reviews academic material is referred to as?...
- C Chemistry Methane gas reacts with oxygen by combustion. How many moles of methane are needed to produce 3.5x10^-4 moles of carbon dioxide?...
- E English After you complete the reading and annotation, answer the following questions in paragraphs (minimum of 3 complete sentences): How do literary elements and rhetorical devices...

Ответ: