radusevciuc7719
12.03.2020 •
Chemistry
If a 95.27 mL sample of acetic acid (HC2H3O2) is titrated to the equivalence point with 79.06 mL of 0.113 M KOH, what is the pH of the titration mixture? (For HC2H3O2 , Ka = 1.82 x 10-5)
Solved
Show answers
More tips
- S Style and Beauty Is Hot Scissor Haircutting Beneficial or Dangerous?...
- S Style and Beauty How to Get Rid of Under Eye Bruises?...
- F Food and Cooking Is Bacon Good for You?...
- S Style and Beauty Discover the Art of Nail Design: How Do You Paint Your Nails?...
- P Philosophy How to Develop Extrasensory Abilities?...
- O Other Everything You Need to Know About Kudyabliks...
- C Computers and Internet The Twitter Phenomenon: What it is and How to Use it...
- C Computers and Internet How to Choose a Laptop: Expert Guide and Tips...
- C Computers and Internet How to Choose a Monitor?...
- H Horoscopes, Magic, Divination Where Did Tarot Cards Come From?...
Answers on questions: Chemistry
- M Mathematics Compare the domains of the logarithmic function ƒ(x) and the square root function g(x). g(x) = squareroot of (3 +1 )+ 1...
- G Geography Rocks that contain crystals that are roughly equal in size and can be identified with the unaided eye are said to exhibit a texture....
- C Chemistry Adeficiency in zinc might result in stunted growth.a deficiency in zinc might result in stunted growth. true...
- C Chemistry Is melting sugar a chemical or physical change? Please provide an explanation...

Ответ:
8.73
Explanation:
The concentration of acetic acid can be determined as follows:
Moles of =
= 
=0.0090 moles
Moles of
= 0.0090 moles
The equation for the reaction can be expressed as :
Concentration of ion =
ion = 
=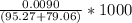
= 0.052 M
Hydrolysis of ion:
ion:
⇒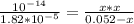
=
As K is so less, then x appears to be a very infinitesimal small number
0.052-x ≅ x
pH = 14 - pOH
pH = 14 - 5.27
pH = 8.73
Hence, the pH of the titration mixture = 8.73
Ответ:
Explanation:
A homomonocyclic compound composed of eight sulfur atoms. ...