damienwoodlin6
06.08.2019 •
Chemistry
In the haber process for ammonia synthesis, k " 0.036 for n 2 (g) ! 3 h 2 (g) ∆ 2 nh 3 (g) at 500. k. if a 2.0-l reactor is charged with 1.42 bar of n 2 and 2.87 bar of h 2 , what will the equilibrium partial pressures in the mixture be?
Solved
Show answers
More tips
- H Health and Medicine How to Get Pregnant Faster?...
- S Style and Beauty Lamination of Hair: How it Works and What it is?...
- C Computers and Internet Best Applications for Your iPad: Review of the Best Candidates for Installation...
- S Style and Beauty Intimate Haircut: The Reasons, Popularity, and Risks...
- A Art and Culture When Will Eurovision 2011 Take Place?...
- S Style and Beauty How to Choose the Perfect Hair Straightener?...
- F Family and Home Why Having Pets at Home is Good for Your Health...
- H Health and Medicine How to perform artificial respiration?...
- H Health and Medicine 10 Tips for Avoiding Vitamin Deficiency...
- F Food and Cooking How to Properly Cook Buckwheat?...
Answers on questions: Chemistry
- M Mathematics Number 10 please ???????...
- G Geography What major landform has been created by the interaction of the Eurasian and African plates...
- C Chemistry Use Lewis theory to determine the chemical formula for the compound formed between Al and O....
- E English HEL HURR NO Which uses the best interrogative pronoun to complete the sentence? is going to help me clean and cook all these fish?WhoWhomWhoseWhich...

Ответ:
Answer : The partial pressure of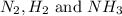 at equilibrium are, 1.133, 2.009, 0.574 bar respectively. The total pressure at equilibrium is, 3.716 bar
at equilibrium are, 1.133, 2.009, 0.574 bar respectively. The total pressure at equilibrium is, 3.716 bar
Solution : Given,
Initial pressure of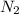 = 1.42 bar
= 1.42 bar
Initial pressure of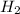 = 2.87 bar
= 2.87 bar
The given equilibrium reaction is,
Initially 1.42 2.87 0
At equilibrium (1.42-x) (2.87-3x) 2x
The expression of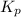 will be,
will be,
Now put all the values of partial pressure, we get
By solving the term x, we get
From the values of 'x' we conclude that, x = 3.889 can not more than initial partial pressures. So, the value of 'x' which is equal to 3.889 is not consider.
Thus, the partial pressure of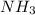 at equilibrium = 2x = 2 × 0.287 = 0.574 bar
at equilibrium = 2x = 2 × 0.287 = 0.574 bar
The partial pressure of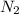 at equilibrium = (1.42-x) = (1.42-0.287) = 1.133 bar
at equilibrium = (1.42-x) = (1.42-0.287) = 1.133 bar
The partial pressure of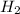 at equilibrium = (2.87-3x) = [2.87-3(0.287)] = 2.009 bar
at equilibrium = (2.87-3x) = [2.87-3(0.287)] = 2.009 bar
The total pressure at equilibrium = Partial pressure of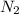 + Partial pressure of
+ Partial pressure of 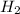 + Partial pressure of
+ Partial pressure of 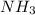
The total pressure at equilibrium = 1.133 + 2.009 + 0.574 = 3.716 bar
Ответ:
x=B/7-6/7
Step-by-step explanation: