davienwatson8
27.05.2021 •
Chemistry
Magnesium reacts with hydrochloric acid to
produce magnesium chloride and hydrogen. In
another investigation, a student reacted 20cm
of hydrochloric acid with a concentration of
1.5 mol dm-3 with excess magnesium.
1) Write a balanced chemical equation for the
reaction.
11) Calculate the number of moles of hydrochloric
acid used.
111) Determine the number of moles of magnesium
chloride produced.
iv) Calculate the mass of magnesium chloride
produced.
Solved
Show answers
More tips
- H Health and Medicine How to Get Pregnant Faster?...
- S Style and Beauty Lamination of Hair: How it Works and What it is?...
- C Computers and Internet Best Applications for Your iPad: Review of the Best Candidates for Installation...
- S Style and Beauty Intimate Haircut: The Reasons, Popularity, and Risks...
- A Art and Culture When Will Eurovision 2011 Take Place?...
- S Style and Beauty How to Choose the Perfect Hair Straightener?...
- F Family and Home Why Having Pets at Home is Good for Your Health...
- H Health and Medicine How to perform artificial respiration?...
- H Health and Medicine 10 Tips for Avoiding Vitamin Deficiency...
- F Food and Cooking How to Properly Cook Buckwheat?...
Answers on questions: Chemistry
- C Chemistry How would you convert 500 kg to ng?...
- C Chemistry Determine the number of significant digits in each of the following...
- C Chemistry To draw a primary amine, start by drawing the longest carbon atom chain of the molecule. Next, connect the amine functional group according to the location given in the name...
- C Chemistry Which of the following cannot be considered a single phrase...
- C Chemistry Determine the molecular weight of h2o. 18 g 18.0 g 18.01 g 18.016 g...
- C Chemistry What are the properties of magnesium and oxygen...
- C Chemistry During lab investigation, robert was given 5 samples of different metals. each sample was the same color and had the same volume. he was asked to determine which of the 5...
- C Chemistry 635 mL of a gas is at apressure of .50 atm. What is the volume of the gas at 1 atm?...
- C Chemistry If a sample of gas occupies 10.0 L at 100 K, what will be its volume in liters be at 300 K if the pressure does not change?...
- C Chemistry 4. Charles law describes the relationship between which two quantities? A.pressure and volume B.miles and pressure C. Volume and temperature D.temperature and moles...

Ответ:
The time required to reach the center line temperature of 800 K is t = 58 sec
Explanation:
Given data
D = 0.1 m
h = 100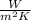
Specific heat for carbon steel (C) = 502.4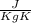
Furnace temperature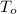 = 1200 K
= 1200 K
Final temperature T = 800 K
Initial temperature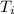 = 300 K
= 300 K
From lumped heat analysis
Now from equation (1)
㏑ 0.44 = -(0.001415 ) t
-(0.001415 ) t = - 0.82
t = 58 sec
Thus the time required to reach the center line temperature of 800 K is
t = 58 sec