treypickich14
22.03.2021 •
Chemistry
molecular formula citric acid2.A 10.0 mL sample of pineapple juice was titrated with 0.100 M sodium hydroxide solution. The average volume of NaOH required to reach the endpoint was 12.8 mL. a.Calculate the number of moles of sodium hydroxide required to reach the endpoint. Show your work in equation editor. Remember to use units and report your answer to the proper number of significant figures.
Solved
Show answers
More tips
- F Family and Home How to Build a Strong Relationship with Your Child: Tips for Effective Communication...
- H Health and Medicine Boosting Immunity: A Complete Guide on How to Improve Your Body’s Natural Defenses...
- C Computers and Internet The Best Antivirus Programs for your PC...
- S Style and Beauty How to Get Rid of Acne: Scientifically Proven Methods...
- H Health and Medicine Simple Ways to Lower Cholesterol in the Blood: Tips and Tricks...
- O Other How to Choose the Best Answer to Your Question on The Grand Question ?...
- L Leisure and Entertainment History of International Women s Day: When Did the Celebration of March 8th Begin?...
- S Style and Beauty Intimate Haircut: The Reasons, Popularity, and Risks...
- A Art and Culture When Will Eurovision 2011 Take Place?...
- S Style and Beauty How to Choose the Perfect Hair Straightener?...
Answers on questions: Chemistry
- C Chemistry Which pair of processes involves the use of bacteria?...
- C Chemistry NEED HELP ASAP Select the correct answer from each drop-down menu. A certain species of fish (A) preys on a smaller species of fish (B). The smaller species of fish mostly consumes...
- C Chemistry An equation that represents the relationship between b and e...
- C Chemistry Calculate the mass percent by volume of 330.1 g of glucose (C₆H₁₂O₆, MM = 180.2 g/mol) in 325 mL of solution....
- C Chemistry Predict the two most likely mechanisms for the reaction of 1-iodohexane with potassium tert-butoxide. A) E1 and SN1 B) SN1 and SN2 C) SN2 and E2 D) E1 and E2...
- C Chemistry Which one of the following best represents the predicted approximate chemical shift and coupling for the hydrogen(s) indicated with the arrow? a) 5.40 ppm, doublet b) 3.00 ppm,...
- C Chemistry For the dissolution of LiCl in water, ΔHsoln=−37kJ/mol. Which term would you expect to be the largest negative number: ΔHsolvent, ΔHsolute, or ΔHmix? a. ΔHsolvent b. ΔHsolute c....
- M Mathematics How do i solve 2 1/3 - 1 3/4...
- S Social Studies 1. La corteza continental es la capa superficial habitad por el se humano ; esta constituida por dos capas llamadas : A . Manto superior e inferior B. Núcleo y manto C . Todas...
- M Mathematics A square has an area of 44 ft. what is the length of each side?...

Ответ:
The number of moles of sodium hydroxide required to reach the endpoint is 0.00128
Explanation:
To calculate the number of moles for given molarity, we use the equation:
Molarity of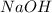 solution = 0.100 M
solution = 0.100 M
Volume of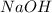 solution = 12.8 mL
solution = 12.8 mL
Putting values in equation, we get:
Thus the number of moles of sodium hydroxide required to reach the endpoint is 0.00128
Ответ: