keva1p6dk26
25.03.2021 •
Chemistry
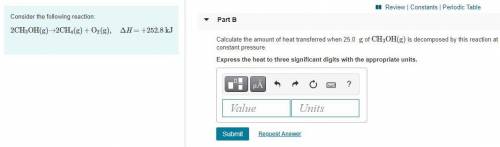
Part B:
Calculate the amount of heat transferred when 25.0 g of CH3OH(g) is decomposed by this reaction at constant pressure. Express the heat to three significant digits with the appropriate units.
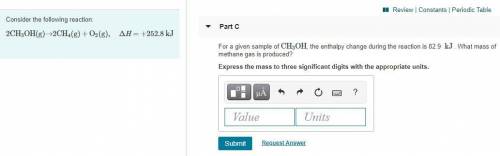
Part C:
For a given sample of CH3OH, the enthalpy change during the reaction is 82.9 kJ. What mass of methane gas is produced? Express the mass to three significant digits with the appropriate units.
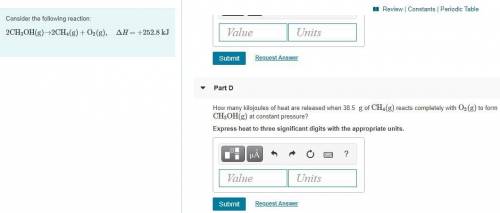
Part D:
How many kilojoules of heat are released when 38.5 g of CH4(g) reacts completely with O2(g) to form CH3OH(g) at constant pressure? Express heat to three significant digits with the appropriate units.
Solved
Show answers
More tips
- C Computers and Internet Can You Insert a Regular SIM Card into an iPad? Let s Find Out...
- F Food and Cooking Лечо: вкусное и простое блюдо для любой кухни...
- H Health and Medicine Relieving Swelling in Legs: Causes and Ways to Alleviate the Symptom...
- S Style and Beauty How to Get Rid of a Bruise: Tips and Tricks...
Answers on questions: Chemistry
- C Chemistry The density of dry air measured at 25 celsius is 1.19 times 10 to the negative 3 what is the volume of 50.0g of air...
- C Chemistry Which of these is correct regarding thomson s cathode ray observations...
- C Chemistry When an ionic bond forms, the atom with the lower ionization energy will be most likely to lose an electron. true false...
- C Chemistry Hey yall my how s ur day?...
- M Mathematics Point A is at (3, - 8) and point M is at (5, - 2.5) . Point M is the midpoint of point A and point B. What are the coordinates of point B?...
- M Mathematics Which expression is equivalent to 32 + 16?...
- E English Which answer best corrects the inappropriate shift in person between the related sentences? Orchestra members are responsible for their instruments. If you damage an instrument,...
- E English Wiesel doesn’t refer to himself and others in the camp as “prisoners”; instead, he says they were “slaves.” Explain why he makes this distinction...
- M Mathematics You measured a piece of paper and you thought the paper was 14 inches long but after you had your friend measure it too you realized the actual paper was 19 inches long. What is...
- E English Can someone please help me with this I don’t understand it I will appreciate your help thank you so much and God bless...

Ответ:
xsahbx
Explanation:
s axnzcxs