cheerthi16
16.10.2019 •
Chemistry
Ph is a logarithmic scale used to indicate the hydrogen ion concentration, [h+], of a solution: ph=−log[h+] due to the autoionization of water, in any aqueous solution, the hydrogen ion concentration and the hydroxide ion concentration, [oh−], are related to each other by the kw of water: kw=[h+][oh−]=1.00×10−14 where 1.00×10−14 is the value at approximately 297 k. based on this relation, the ph and poh are also related to each other as 14.00=ph+poh the temperature for each solution is carried out at approximately 297 k where kw=1.00×10−14. part a 0.35 g of hydrogen chloride (hcl) is dissolved in water to make 2.5 l of solution. what is the ph of the resulting hydrochloric acid solution?
Solved
Show answers
More tips
- C Computers and Internet Отправляем смс через интернет: легко и просто...
- H Health and Medicine Want to Lose Weight? Here s How Many Calories You Need Per Day...
- H Health and Medicine How Many Ribs Do Humans Have?...
- C Computers and Internet Dropbox: What is it and How to Use it...
- F Family and Home Choosing the Right Car Seat for Your Child: Tips and Recommendations...
- C Computers and Internet Е-head: How it Simplifies Life for Users?...
- A Auto and Moto How many blood alcohol level units are allowed in Russian traffic laws?...
- H Horoscopes, Magic, Divination Where Did Tarot Cards Come From?...
- H Health and Medicine How to Deal with Heat Stroke?...
- H Health and Medicine Sunstroke: Causes, Symptoms, and Precautions...
Answers on questions: Chemistry
- C Chemistry QUESTION 25. At the critical temperature, the surface tensionof the liquidOIs zeroO is infinityis the same as that at the othertemperatureO none of these...
- C Chemistry Bi + HgCl2 → Will reaction occur?...
- C Chemistry A truck uses 6 kg of fuel which undergoes complete combustion. The heat produced during the complete combustion was measured to be 220,000 kJ. Calculate the calorific value of...
- C Chemistry What state of matter are the most organized state of matter. Solids Liquids Gases...
- C Chemistry During the summer after your first year at Carnegie Mellon, you are lucky enough to get a job making coffee at Starbucks, but you tell your parents and friends that you have secured...
- C Chemistry All factors that cause an increase in the rate of dissolving have what in common? They cause more frequent collisions between solute and solvent particles. They decrease the kinetic...
- C Chemistry Which element below is the most reactive? Hydrogen chlorine neon oxygen...
- C Chemistry The mobility and carrier density of Al are 12 cm2/Vs and 1.98×1023 cm−3 , respectively. The mobility and carrier density of Cu are 43.2 cm2/Vs and 8.5×1022 cm−3 , respectively....
- M Mathematics The graph models projected populations, in thousand, of two towns after x years. Both towns experience a 1.9% annual growth rate. Which equation represents g(x) as a translation...
- M Mathematics Please help!! I don t understand this...

Ответ:
pH = 2,4
Explanation:
pH is defined as a measure of acidity or alkalinity of a solution based on H⁺ concentration
The HCl dissociates in water thus:
HCl → H⁺ + Cl⁻
That means that moles of HCl are the same than H⁺.
0,35gHCl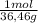 = 9,6x10⁻³ moles HCl ≡ moles H⁺
= 9,6x10⁻³ moles HCl ≡ moles H⁺
H⁺ molarity is: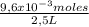 = 3,84x10⁻³M
= 3,84x10⁻³M
As pH = -log [H⁺]
pH = 2,4
I hope it helps!
Ответ:
Just add a plenty of dots in the first one and very few dots in the second one