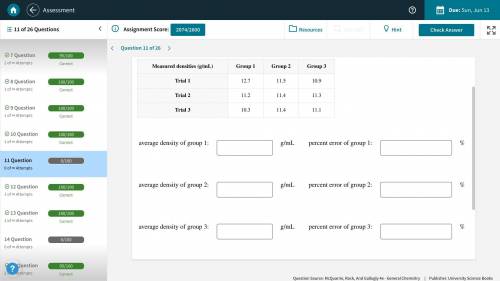
(Picture Included) A handbook lists the density of lead as 11.3 g/mL. Several groups of students are attempting to determine the density of a lead weight by various methods. Calculate the average density measured by each group, and the percentage error in each average.
Solved
Show answers
More tips
- S Style and Beauty Discover the Art of Nail Design: How Do You Paint Your Nails?...
- P Philosophy How to Develop Extrasensory Abilities?...
- O Other Everything You Need to Know About Kudyabliks...
- C Computers and Internet The Twitter Phenomenon: What it is and How to Use it...
- C Computers and Internet How to Choose a Laptop: Expert Guide and Tips...
- C Computers and Internet How to Choose a Monitor?...
- H Horoscopes, Magic, Divination Where Did Tarot Cards Come From?...
- S Style and Beauty How to Make Your Lips Fuller? Ideas and Tips for Beautiful Lips...
- C Computers and Internet How to Learn to Type Fast?...
Answers on questions: Chemistry
- C Chemistry 2.92 A 50.0-g silver object and a 50.0-g gold object are both added to 75.5 mL of water contained in a graduated cylinder. What is the new water level in the cylinder?...
- C Chemistry How do you know a physical change has occurred when evaporating water produces gaseous water? A A chemical change has resulted in a new substance. B A chemical change...
- C Chemistry Does heroin change your appetite? and what kind of drug is it...
- C Chemistry Calculate the pH at the equivalence point in titrating 0.100 M solutions of the following with 0.080M NaOH: hydrobromic acid (HBr)...
- B Biology Gametes are cells produced in vascular plants that are necessary for the plant to carry out what function? A)Transportation B)Reproduction C)Photosynthesis D)Geotropism...
- B Biology Un ejemplo de una adaptación que se encuentra en tres plantas o animales diferentes. Construya una explicación de cómo la adaptación ayuda a cada organismo a sobrevivir...
- S Social Studies The rise of democratic and individualistic beliefs, a response to rationalism, and changes to society caused by the market revolution, along with greater social and...
- M Mathematics If you use paper to solve 521 - 39, what is an important thing to conside 1. You subtract 9 - 1 to get 8 2. You really need a calculator since this is too hard 3.You...
- S Social Studies Immigrants fleeing famine or joblessness in their home countries came to the United States because...
- P Physics In a system, when energy is transformed from one form to another O A. some energy is always destroyed. O B. new energy is created. O c. the total energy is conserved...

Ответ:
ask the teacher then dummy
Explanation: