mxrvin1350
28.06.2019 •
Chemistry
Potassium hydroxide (koh) reacts with sulfuric acid (h2so4) to produce potassium sulfate (k2so4) and water. what best describes this reaction? a single replacement reaction occurs because potassium ions bond with sulfate ions to form a salt. a double replacement reaction occurs because potassium ions bond with sulfate ions to form a salt. a single replacement reaction occurs because potassium is more reactive than hydrogen. a double replacement reaction occurs because potassium is less reactive than hydrogen.
Solved
Show answers
More tips
- C Computers and Internet IMHO: What is it, why is it important, and how to use it?...
- H Health and Medicine How to Calculate Your Ideal Weight?...
- S Style and Beauty Discover the Art of Nail Design: How Do You Paint Your Nails?...
- P Philosophy How to Develop Extrasensory Abilities?...
- O Other Everything You Need to Know About Kudyabliks...
- C Computers and Internet The Twitter Phenomenon: What it is and How to Use it...
- C Computers and Internet How to Choose a Laptop: Expert Guide and Tips...
- C Computers and Internet How to Choose a Monitor?...
- H Horoscopes, Magic, Divination Where Did Tarot Cards Come From?...
- S Style and Beauty How to Make Your Lips Fuller? Ideas and Tips for Beautiful Lips...
Answers on questions: Chemistry
- C Chemistry Please answer this and explain why :)...
- B Business A is a word, name, symbol, or device that is used in trade with goods to indicate the source of the goods and to distinguish them from the goods of others....
- H History Suppose the height of a cylinder is equal to its radius. the cylinder can fit inside a square prism, as shown below the cross-sectional areas are still the same and the ratio...
- M Mathematics the table and graph shows how many words morgan and brian typed correctly on a typing test. for both students the relationship between words type correctly and time is linear...
- M Mathematics Abakery sold 108 cupcakes in one day. the head baker predicted he would sell 88 cupcakes that day. what was the percent error of the baker s prediction?...
- M Mathematics Which function is represented by the graph? 5 4 3 2 f(x) = -x - 3] + 4 f(x) = -x + 3] + 4 f(x) = -|* - 4 + 3 f(x) = -x + 4 + 3 1 5 4 3 2 2-1,4 1 2 3 4 5 -27 -3 9 T4...

Ответ:
Option (b) is the correct answer.
Explanation:
The given reaction is as follows.
In this reaction, potassium ions form bond with sulfate ions and results in the formation of potassium sulfate. Similarly, hydroxide ions combine with hydrogen ions to result in the formation of water.
Thus, we can conclude that it is a double replacement reaction occurs because potassium ions bond with sulfate ions to form a salt.
Ответ:
A double replacement reaction occurs because potassium ions bond with sulfate ions to form a salt.
Explanation:
A double replacement reaction is defined as chemical reaction in which ions of two reactant interchange their position with each other to give out product.
Here, in question:
In the above chemical reaction, when potassium hydroxide reacts with sulfuric acid, the interchanging of ions takes place in between the reactants that is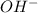 ion of KOH combines with
ion of KOH combines with 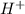 ion of sulfuric acid to give water.
ion of sulfuric acid to give water.
Also sulfate ion (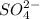 ) of sulfuric acid gets combine with two potassium ion (
) of sulfuric acid gets combine with two potassium ion (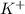 )to give salt of potassium sulfate,
)to give salt of potassium sulfate, 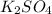 .
.
Hence , it will be correct to say,A double replacement reaction occurs because potassium ions bond with sulfate ions to form a salt.
Ответ:
B.) weather
Explanation:
weather is constantly changing everyday and each day presents a new condition:)