riverviewfarm2133
13.02.2021 •
Chemistry
Students in chemistry class have been given the assignment to use flame test emission data to determine the identity of an
unknown substance. When the students put a sample of the unknown compound in the flame, blue and green colors were visible.
What could be one explanation for these results?
A)
The Bunsen burner must not be working properly.
B)
The unknown substance must be a sodium compound.
The unknown substance contained both copper and lead.
D)
It is impossible to narrow down anything about the unknown
Solved
Show answers
More tips
- C Computers and Internet Protect Your Computer: How to Set a Password on Your Device...
- W Work and Career How to behave at an interview? Tips from an HR specialist...
- F Family and Home How to Keep Your Home Warm: Tips and Tricks...
- D Dating, Love, Relationships Does a Person s Character Depend on the Color of Their Eyes?...
- O Other Childhood Fears: What Many of Us Experienced...
- H Health and Medicine Simple and Effective: How to Get Rid of Cracked Heels...
- O Other How to Choose the Best Answer to Your Question on The Grand Question ?...
- L Leisure and Entertainment History of International Women s Day: When Did the Celebration of March 8th Begin?...
- S Style and Beauty Intimate Haircut: The Reasons, Popularity, and Risks...
- A Art and Culture When Will Eurovision 2011 Take Place?...
Answers on questions: Chemistry
- M Mathematics Find x in the following two irregular similar polygons. a.17.5 b.18 c.18.5 d.19...
- M Mathematics A chemical consists of four compounds,A,B,C and D. 1 2 and D. 1/6 is A,2/5 is B,1/10 is C and the rest is D.What fraction of the chemical is D?...
- M Mathematics To move a function, you need to it....
- S Spanish 2. Tienes que contarlos chistes. - Les tienes que contar chistes a los amigos b. a todas las chicas c. a Mariela y Diana...
- G Geography Base your answer on the passage below and on your knowledge of social studies The period of imperialism has witnessed many wars. Most of these wars have been caused by attacks of...

Ответ:
1.10 g H₂
Explanation:
The reaction is
2HCl + Mg → MgCl₂ + H₂
An excess of magnesium metal means that the limiting reactant is HCl, so we use the mass of HCl given by the problem to calculate how many grams of H₂ are produced.
In order to make this calculation we need to keep in mind the molar mass of HCl, of H₂, and the reaction stoichiometry:
40.0 g HCl *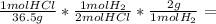 1.10 g H₂
1.10 g H₂