naomicervero
15.04.2021 •
Chemistry
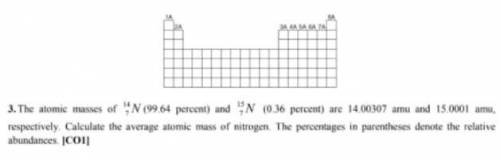
The atomic musses of N (99. 64 percent) and 1N (0.36 percent) are 14.00307 amu and 15.0001 amu, respectively. Calculate the average atomic mass of nitrogen. The percentages in parentheses denote the relative abundances C01?
Solved
Show answers
More tips
- A Animals and plants How to Properly Care for a Pet Decorative Rabbit at Home?...
- C Computers and Internet How to Check the Speed of My Internet?...
- H Health and Medicine 10 Ways to Cleanse Your Colon and Improve Your Health...
- W Work and Career How to Write a Resume That Catches the Employer s Attention?...
- C Computers and Internet Е-head: How it Simplifies Life for Users?...
- F Family and Home How to Choose the Best Diapers for Your Baby?...
- F Family and Home Parquet or laminate, which is better?...
- L Leisure and Entertainment How to Properly Wind Fishing Line onto a Reel?...
- L Leisure and Entertainment How to Make a Paper Boat in Simple Steps...
- T Travel and tourism Maldives Adventures: What is the Best Season to Visit the Luxurious Beaches?...

Ответ:
14.01 amu
Explanation:
Step 1: Given data
Isotope Atomic mass (am) Abundance (ab)
¹⁴N 14.00307 amu 99.64% (0.9964)
¹⁵N 15.0001 amu 0.36% (0.0036)
Step 2: Calculate the average atomic mass (aam) of nitrogen
We will use the following expression.
aam = ∑ am × ab
aam = 14.00307 amu × 0.9964 + 15.0001 amu × 0.0036 = 14.01 amu
Ответ:
Steeper:
y= -2x+7/3
y=-7/4x + 3/2
Less steep:
y=-8/7x + 4/7
y=-3/2x+1/2
y=-4/3x+2/3
Hope this is helpful!