leahmitch612
18.10.2019 •
Chemistry
The chemical equation for a reaction between k2cr2o7 and hcl is shown. k2cr2o7 + 14hcl → 2crcl3 + 2kcl + 3cl2 + 7h2o
which of the following identifies the reactant that acts as an oxidizing agent in the reaction and explains the answer? 20 !
a. k2cr2o7, because the oxidation number of k changes from +6 to +3.
b. k2cr2o7, because the oxidation number of cr changes from +6 to +3.
c. hcl, because the oxidation number of h changes from −1 to 0.
d. hcl, because the oxidation number of cl changes from −1 to 0.
Solved
Show answers
More tips
- F Family and Home How to Build a Strong Relationship with Your Child: Tips for Effective Communication...
- H Health and Medicine Boosting Immunity: A Complete Guide on How to Improve Your Body’s Natural Defenses...
- C Computers and Internet The Best Antivirus Programs for your PC...
- S Style and Beauty How to Get Rid of Acne: Scientifically Proven Methods...
- H Health and Medicine Simple Ways to Lower Cholesterol in the Blood: Tips and Tricks...
- O Other How to Choose the Best Answer to Your Question on The Grand Question ?...
- L Leisure and Entertainment History of International Women s Day: When Did the Celebration of March 8th Begin?...
- S Style and Beauty Intimate Haircut: The Reasons, Popularity, and Risks...
- A Art and Culture When Will Eurovision 2011 Take Place?...
- S Style and Beauty How to Choose the Perfect Hair Straightener?...
Answers on questions: Chemistry
- C Chemistry When baking soda and vinegar are mixed, how does sodium acetate form from this chemical reaction?...
- C Chemistry is a chemical process within cells where sugar and oxygen react to form carbon dioxide and water resulting in the release of energy ASAP ANSWER...
- C Chemistry Balance and classify the equation: H2SO4 = H2 + S03...
- C Chemistry A scientist measures the mass of two liquids before and after combining them. The mass after combining the liquids is less than the sum of the masses before. Where is the...
- C Chemistry A concrete floor is poured as a liquid. As it hardens, it produces quite a bit of heat. Physical change or chemical change?...
- C Chemistry Determine the charge on the unknown ion, X, in the compound AlX3....
- C Chemistry 0.025 millimeters is the same as how many liters?...
- C Chemistry Question 5 (2 points) Which of the following has a higher boiling point, CO2 or Co? Explain....
- C Chemistry Identify the variable that represents the number of particles added. Identify the variable that represents the pressure added to the syringe....
- C Chemistry How are technological advancements changing the way that we think about measurement?...

Ответ:
The correct answer is Option b.
Explanation:
Oxidizing agent is defined as the chemical reagent which helps the other chemical compound to get oxidized and itself gets reduced. The oxidation state for these species gets reduced because they are undergoing reduction reaction.
For the given chemical equation:
Oxidation state of Chromium is getting reduced from +6 to +3 and oxidation state of chlorine getting increased from -1 to 0.
Hence, acts like and oxidizing agent because it is itself getting reduced to
acts like and oxidizing agent because it is itself getting reduced to 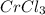
Therefore, the correct answer is Option b.
Ответ:
The self-ionization of water is an ionization reaction in pure water or in an aqueous solution, in which a water molecule, H₂O, deprotonates to become a hydroxide ion, OH⁻. The hydrogen nucleus, H⁺, immediately protonates another water molecule to form hydronium, H₃O⁺.
Equation : In the attachment.
Hope it helps.