The electron pair in a C-F bond could be considered Question 3 options: closer to C because carbon has a larger radius and thus exerts greater control over the shared electron pair closer to C because carbon has a lower electronegativity than fluorine an inadequate model since the bond is ionic centrally located directly between the C and F closer to F because fluorine has a higher electronegativity than carbon
Solved
Show answers
More tips
- P Philosophy How did the concept of module arise in computer science?...
- F Food and Cooking How to Cook Julienne? Recipes and Tips...
- H Health and Medicine At What Age Does a Person Stop Growing?...
- F Family and Home How to Choose a Name for Your Baby?...
- F Food and Cooking Discover the Health Benefits of Cedar Nuts...
- L Leisure and Entertainment Scrapbooking: What is it and Why is it Becoming More Popular?...
- L Leisure and Entertainment Carving: History and Techniques for Creating Vegetable and Fruit Decorations...
- F Food and Cooking How to Make Sushi: A Step-by-Step Guide to Perfectly Rolled Delights...
- T Travel and tourism Maldives Adventures: What is the Best Season to Visit the Luxurious Beaches?...
- F Food and Cooking Аэрогриль: Все, что нужно знать для подготовки здоровой и вкусной пищи...
Answers on questions: Chemistry
- B Business Bostian, inc. has total assets of $575,000. its total debt outstanding is $185,000. the board of directors has directed the cfo to move towards a debt-to-assets ratio of 55%....
- M Mathematics How can you write one and 16,000’s as a decimal...
- S Social Studies Q1: about how many miles is the mississippi river q2: in witch state the the mississippi river begin q3: in witch state does the mississippi river end q4: lablel the bodies...

Ответ:
closer to F because fluorine has a higher electronegativity than carbon
Explanation:
Electronegativity refers to the ability of an atom in a bonding situation to draw the shared electrons of the bond closer to itself.
Electronegativity increases across the period and decreases down the group. A highly electronegative atom draws the shared electron pair of a bond towards itself.
When two atoms are bonded together, the electron pair is always drawn closer to the atom that has a higher electronegativity.
Hence, the electron pair in a C-F bond could be considered closer to F because fluorine has a higher electronegativity than carbon.
Ответ:
2.67 x 10⁷km
Explanation:
Given parameters:
Time of arrival of p-waves = 2: 10: 00
Time of arrival of s-waves = 2: 15: 20
Unknown:
Distance of wave from epicenter = ?
Solution:
To find the distance of the wave from the epicenter;
Distance = 3 x 10⁸ x time interval
Time interval = Time of arrival of p-waves - Time of arrival of s-waves
= 2: 10: 00 - 2: 15: 20
= 5min 20s
= 320s
Now, we need to convert the time to hrs;
3600s = 1hr
320s =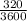 =
= 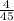 hr
hr
So,
Distance = 3 x 10⁸ x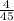 = 2.67 x 10⁷km
= 2.67 x 10⁷km