The equilibrium constant, kc, for the following reaction is 5.10×10-6 at 548 k. nh4cl(s) nh3(g) + hcl(g) calculate the equilibrium concentration of hcl when 0.564 moles of nh4cl(s) are introduced into a 1.00 l vessel at 548 k.[hcl] = m
Solved
Show answers
More tips
- G Goods and services What Are the Most Popular Services?...
- O Other What is the oldest joke ever told?...
- L Legal consultation How to Properly Inherit: Tips and Recommendations...
- C Computers and Internet Boost your processor performance with these easy tips...
- S Sport How does Bodyflex work: what is it and how does it work?...
- H Health and Medicine How to Whiten Teeth and Get the Perfect Smile...
- S Style and Beauty How to Properly Apply Eye Makeup: Tips from a Professional Makeup Artist...
- A Auto and Moto How Can Parking Sensors Help Drivers?...
- C Computers and Internet Make Money Online: Secrets and Essential Ways...
- A Auto and Moto What is the Average Lifespan of an Engine in a Car?...
Answers on questions: Chemistry
- C Chemistry If 1.00 mol of cs2 is combined with 1.00 mol of o2, identify the limiting reactant...
- C Chemistry Ca(s)+br2(l)⟶cabr2(s) express your answer as a chemical equation. identify all of the phases in your answer....
- C Chemistry A211 g sample of barium carbonate, baco3, reacts with a solution of nitric acid to give barium nitrate, carbon dioxide, and water. if the acid is present in excess,...
- C Chemistry If an atom has a diameter of 395 pm, what is its radius?...
- C Chemistry How is the effectiveness of soap impacted by solvent ?...
- E English . Which word is a verbal known as a gerund?...
- S Spanish Is this sentence correct ? ELLA VIAJA CON MI...
- M Mathematics Twice the sum of consecutive numbers n and is 510....
- S Social Studies As an employee of the FDA Jamal is notified that there have been several reported cases of illness in a three-county area. Doctors have found that all the people who...
- H Health What is Biophotonics used for in today s society? (this is for science)...

Ответ:
The equilibrium concentration of HCl is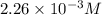
Explanation:
We are given:
Moles of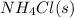 = 0.564 moles
= 0.564 moles
Volume of vessel = 1.00 L
Molarity is calculated by using the equation:
Molarity of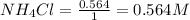
The given chemical equation follows:
Initial: 0.564
At eqllm: 0.564-x x x
The expression of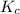 for above equation follows:
for above equation follows:
The concentration of pure solid and pure liquid is taken as 1.
We are given:
Putting values in above equation, we get:
Negative sign is neglected because concentration cannot be negative.
So,![[HCl]=2.26\times 10^{-3}M](/tpl/images/0405/9498/283fd.png)
Hence, the equilibrium concentration of HCl is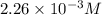
Ответ: