Anonymouslizard
13.03.2020 •
Chemistry
The following initial rate data apply to the raction
F2(g) + 2Cl2O(g) ---> 2FClO2(g) +Cl2(g)
Expt. [F2] (M) [Cl2O] (M) Intitial rate (M/s)
1 0.05 0.010 5 x 10^-4
2 0.05 0.040 2.0 x 10^-3
3 0.10 0.010 1.0 x 10^-3
Which of the following is the rate law (rate equation) for this reaction?
A. rate= k[F2]^2 [Cl2O]^4
B. rate= k[F2]^2 [Cl2O]
C. rate= k[F2] [Cl2O]
D. rate= k[F2] [Cl2O]^2
E. rate= k[F2]^2 [Cl2O]^2
Please show work
Solved
Show answers
More tips
- C Computers and Internet How to Get Rid of 3pic Infector: Everything You Need to Know...
- A Auto and Moto Experience the World of the Most Expensive Cars on the Planet...
- S Style and Beauty How to Get Rid of a Double Chin?...
- F Food and Cooking How to Cook Julienne? Recipes and Tips...
- D Dating, Love, Relationships 10 Useful Tips on How to Survive a Breakup?...
- F Food and Cooking Apple Cider Vinegar: The Ultimate Health and Beauty Solution...
- C Computers and Internet Е-head: How it Simplifies Life for Users?...
- F Family and Home How to Choose the Best Diapers for Your Baby?...
- F Family and Home Parquet or laminate, which is better?...
- L Leisure and Entertainment How to Properly Wind Fishing Line onto a Reel?...
Answers on questions: Chemistry
- C Chemistry the students in ms. green s science class dissolved four salts in water. students varied water temperature to see if more or less salt would dissolve in a constant...
- C Chemistry Carole owns a two story house with a heating and cooling unit on each floor. in the winter she mostly uses the downstairs unit because the volume of warm air and warming...
- C Chemistry Each element in the periodic table is assigned an atomic number. this number is the same as?...
- C Chemistry How is a car polluting the earth...
- C Chemistry Kevin decided to measure the amount of carbon and some primary greenhouses gases in the atmosphere . The results of his measurement showed a high carbon footprint and...
- C Chemistry Use the following information to answer the next question. Instead of the hydrogen half-cell, the cobalt half-cell is used as the reference cell where its standard...
- E English Can the government make a decision for gun control...
- E English How might an after school job be used to enhance, rather than limit, future opportunities for teenagers?...
- M Mathematics In a family with 7 children, excluding multiple births, what is the probability of having 6 boys and 1 girl, in any order...
- B Biology Anursing assessment for a client with alcohol abuse reveals a disheveled appearance and a foul body odor. what is the best initial nursing plan that would assist the...

Ответ:
The given information gives us the clue that deuterium and tritium react together. We need to find the balanced nuclear reaction of deuterium and tritium; however, first, let me explain you what deuterium and tritium are.
Deuterium and tritium are two isotopes of hydrogen having atomic mass of 2 and 3 respectively. Deuterium is represented by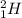 , whereas tritium is written as
, whereas tritium is written as 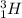 .
.
When deuterium and tritium collide together, they form Helium atom, which is written as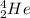 , and also release energy (and shoot out one neutron).
, and also release energy (and shoot out one neutron).
Hence, the balanced nuclear reaction will be the following:
-i