jak000067oyyfia
20.12.2021 •
Chemistry
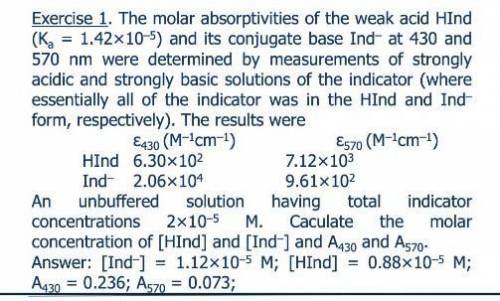
The molar absorptivities at 430 and 570 nm of the weak acid HIn (Ka = 1.42 ? 10-5) and its conjugate base In- were determined by measurements of strongly acidic and strongly basic solutions of the indicator. Under this conditions, essentially all of the indicator was in the HIn and In- form, respectively. The molar ab-sorptivities of HIn and In- at 430 nm and 570 nm were 6.30 ? 102 and 7.12 ? 103, and 2.06 ? 104 and 9.61 ? 102, respectively. Calculate absorbance data for unbuffered solutions that have total indicator concentrations ranging from 2 ? 10-5 to 16 ? 10-5 M.
Solved
Show answers
More tips
- S Science and Technology The Metric System in Our Daily Life: Understanding Its Importance...
- H Health and Medicine Angina: Causes, Symptoms, and Treatment...
- C Computers and Internet How to Learn to Type Fast?...
- F Food and Cooking Delight for Gourmets: How to Prepare Liver Pate...
- S Style and Beauty How to braid friendship bracelets?...
- H Health and Medicine Mercury Thermometer Danger: What to do when a thermometer breaks?...
- F Food and Cooking Which Calamari Salad is the Most Delicious?...
- S Society and Politics 10 Tips for Boosting Your Self-Esteem...
- F Food and Cooking The Most Delicious and Simple Fish in Batter Recipe...
- H Health and Medicine What is Autism? Understanding the Basics of This Neurodevelopmental Disorder...
Answers on questions: Chemistry
- C Chemistry My breasts are really sensitive, and depending on when my partner touches them, this can make or break a pleasure session. What connection do breast sensations have to sexual...
- C Chemistry What does volatility refer to? O A. The tendency of liquids to boil OB. The tendency of liquids to form vapors O C. The tendency of liquids to form solids D. The tendency...
- C Chemistry A 38.2g sample of hydrogen gas, H, is placed into a 2-L soda bottle. IfI keep the pressure and temperature constant, how large would my container need to be if I increased...
- C Chemistry KCIO3(s) → KCl(s) + O2(g) in word equation...
- C Chemistry Suppose you wanted to produce 1.00 L of 3.50 M solution of H2SO4. How many grams of the solute are needed to make this solution?...
- C Chemistry Should diesel and gasoline engines compliant with co2 emission standards be banned?...
- C Chemistry Which statement correctly describes the condensationof a gas?...
- C Chemistry What MOSTLY determines the chemical properties of the atoms of an element? A) proton number B) neutron number C) electron number D) valence electron number...
- C Chemistry If a scientist knows the relative age of a fossil, he can also know the relative age of the rock where it was found. how long the organism was alive. how it became extinct....
- C Chemistry Asolution that has a poh of 5.36 is- a. cannot be determined b. acidic c.neutral d. basic...

Ответ:
Be a big brain
Explanation: