deonnaturner68p7hz7y
05.12.2019 •
Chemistry
The overall reaction 2co3+(aq) + 2cl–(aq) → 2co2+(aq) + cl2(g) has the standard cell voltage e°cell = 0.46 v. given e° = 1.36 v for the reaction cl2(g) + 2e– → 2cl–(aq), calculate the standard reduction potential for the following the half reaction at 25°c: co3+ + e– → co2+
Solved
Show answers
More tips
- W Work and Career Secrets of Punctuality: How to Learn to Never Be Late?...
- L Leisure and Entertainment How to Choose the Perfect Gift for Men on February 23rd?...
- H Health and Medicine How to Treat Whooping Cough in Children?...
- H Health and Medicine Simple Ways to Lower Cholesterol in the Blood: Tips and Tricks...
- O Other How to Choose the Best Answer to Your Question on The Grand Question ?...
- L Leisure and Entertainment History of International Women s Day: When Did the Celebration of March 8th Begin?...
- S Style and Beauty Intimate Haircut: The Reasons, Popularity, and Risks...
- A Art and Culture When Will Eurovision 2011 Take Place?...
- S Style and Beauty How to Choose the Perfect Hair Straightener?...
- F Family and Home Why Having Pets at Home is Good for Your Health...
Answers on questions: Chemistry
- C Chemistry Which of the following substances represents a compound? A. saline: NaCl and H,0 B. helium: H c. carbon dioxide: CO D. iron; Fe...
- C Chemistry On 7 Determine the last significant digit (least certain) in the following measurement: 21 3.01 x 10 ) nplete out of Select one: a. 3 question o b. O C. 1 Check Fini POGIL...
- C Chemistry Mass percent of carbon in hexanol...
- C Chemistry Find the amount of S⁸ molecules, in 3 g of sulfur....
- C Chemistry Solve the following calculation and round to the correct number of significant figures: 34.683 + 58.930 + 68.35112 = if anyone can i would really appreciate it you!...
- C Chemistry Solve the following calculation and round to the correct number of significant figures: 45001 - 56.355 - 78.44 = me you : )...
- C Chemistry Which statement is best supported by the table? levi observed properties of four different waves and recorded observations about each one in his chart. wad it is impossible...
- C Chemistry Me i would really appreciate it you!...
- C Chemistry How can you change the volume of gas being produced by the reaction?...
- C Chemistry Como sacar los protones , neutrones y electrones...

Ответ:
The standard reduction potential for reduction half reaction is 1.82 V
Explanation:
The given chemical reaction follows:
Oxidation half reaction: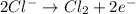
Reduction half reaction: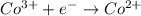
Substance getting oxidized always act as anode and the one getting reduced always act as cathode.
To calculate the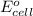 of the reaction, we use the equation:
of the reaction, we use the equation:
We are given:
Putting values in above equation, we get:
Hence, the standard reduction potential for reduction half reaction is 1.82 V
Ответ:
becoz they want so
we cant explain ^^