The p K a of hypochlorous acid is 7.530 . A 60.0 mL solution of 0.103 M sodium hypochlorite ( NaOCl ) is titrated with 0.296 M HCl . Calculate the pH of the solution after the addition of 8.00 mL of 0.296 M HCl .
Solved
Show answers
More tips
- S Science and Technology The Metric System in Our Daily Life: Understanding Its Importance...
- H Health and Medicine Angina: Causes, Symptoms, and Treatment...
- C Computers and Internet How to Learn to Type Fast?...
- F Food and Cooking Delight for Gourmets: How to Prepare Liver Pate...
- S Style and Beauty How to braid friendship bracelets?...
- H Health and Medicine Mercury Thermometer Danger: What to do when a thermometer breaks?...
- F Food and Cooking Which Calamari Salad is the Most Delicious?...
- S Society and Politics 10 Tips for Boosting Your Self-Esteem...
- F Food and Cooking The Most Delicious and Simple Fish in Batter Recipe...
- H Health and Medicine What is Autism? Understanding the Basics of This Neurodevelopmental Disorder...
Answers on questions: Chemistry
- C Chemistry You have an unknown solution in a beaker and you want to test it to see what it is. You put red pH paper in it and it remains red. What do you know about the solution?...
- C Chemistry When was the Grand Canyon formed...
- C Chemistry This chemical paste is very good for keeping houses nice and fresh. It absorbs any bad smells. There are 10 grams of lithium in each teaspoon of the paste. If there are...
- C Chemistry 1. Express the wave speed of each wave described below in meters per second. A wave travels through a 2 meter rope in 1 second....
- C Chemistry What happens when the south end of Earth’s axis is tilted toward the Sun? The Northern Hemisphere experiences fall. The Northern Hemisphere experiences spring. The Southern...
- C Chemistry During a lunar eclipse, the moon turns dark due to the deviation of the sun s rays caused by Earth s atmosphere....
- C Chemistry 5.01 kJ are used to melt a sample of ice at 0°C. What is the mass of the sample? general formula:...
- C Chemistry What causes some metallic bonds to be stronger than others? A.the number of donated valence electrons B.the number of metallic bonds C.the fineness of the metal D.the number...
- M Mathematics Is the function f(x)= sec x and g(x)= sin x, what is the composed function f(g(x)= sec(sin x) and what are its domain and range?...
- P Physics Atoms may be bonded together by losing, gaining, or sharing question 1 options: a, photons b. energy c. electrons d. neutrons e. protons...

Ответ:
pH of the solution after addition of 8 ml 0,296 molar HCl = 7.94
Explanation:
In this case buffer exist between hypochlorous acid HClO is a weak acid and its conjugate base hypochlorite ion ClO⁻ which are deliver to the solution by NaOCl ( sodium hypochlorite ).
The salt is completely dissociate
[NaOCl] = [ClO⁻]
Using Henderson equation to find pH of this buffer
pH = pKa + log![\frac{[Conjugate base]}{[Acid]}](/tpl/images/0559/7901/225c1.png)
or, = 7.53 + log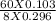
or, = 7.53 + 0.4
or, = 7.94
Ответ:
the formula for heat capacity is q=mct but the formula for specific heat is c= q/mt so to caluclate the specific heat of a metal, you use c=q/mt