deandrehudson18
02.02.2021 •
Chemistry
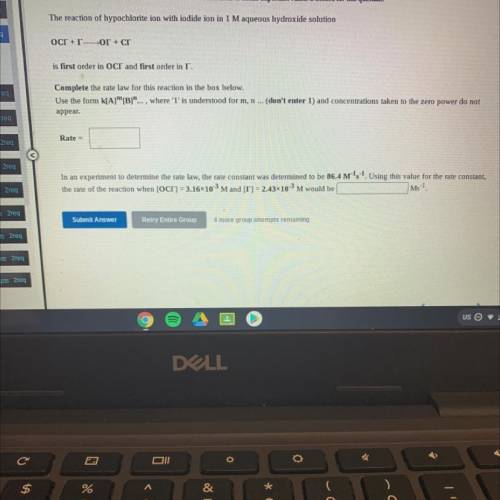
The reaction of hypochlorite ion with iodide ion in 1 M aqueous hydroxide solution
OCT +TOr + cr
is first order in OCT and first order in I.
Complete the rate law for this reaction in the box below.
Use the form k[A]”[B]"..., where 'l' is understood for m, n ... (don't enter 1) and concentrations taken to the zero power do not
appear.
Rate =
In an experiment to determine the rate law, the rate constant was determined to be 86.4 M's-1. Using this value for the rate constant,
the rate of the reaction when [OC] = 3.16*10-3 Mand [T] = 2.43*10-3 M would be
Ms-1
Solved
Show answers
More tips
- G Goods and services How to Choose a Video Camera: Tips from Professionals...
- F Family and Home How to Remove Tar Stains: Tips and Recommendations from Experts...
- H Health and Medicine Novomin: What is it and how to use it?...
- P Philosophy Unbelievable stories of encounters with otherworldly forces...
- L Leisure and Entertainment How to Choose the Perfect Gift for Men on February 23rd?...
- H Health and Medicine How to Treat Whooping Cough in Children?...
- H Health and Medicine Simple Ways to Lower Cholesterol in the Blood: Tips and Tricks...
- O Other How to Choose the Best Answer to Your Question on The Grand Question ?...
- L Leisure and Entertainment History of International Women s Day: When Did the Celebration of March 8th Begin?...
- S Style and Beauty Intimate Haircut: The Reasons, Popularity, and Risks...
Answers on questions: Chemistry
- C Chemistry in a certain electrolysis experiment 1.55 g of Ag were deposited in one cell containing aqueous agno3 sol. calculate the mass of zn deposited in another cell containing aq znso4...
- C Chemistry Which of the following describes how a recycling program directly benefits a community economically? The guarantee of no new landfills or incinerators O Fewer city taxes than...
- E English CAN YOU HELP ME PLEASEEE IS LITERATURE In the context of this story and the greater history of the Inquisition, how does power corrupt? Why does it corrupt? What effects does...
- M Mathematics 7/8y +1/3 = 7/12 what does y equal?...
- P Physics What are the properties of images seen in a concave mirror if the object is farther from the mirror than the focal point?...

Ответ:
The volume of the blood sample is 5mL
HOW TO CALCULATE VOLUME:
The volume of a blood sample can be calculated by dividing the mass (g) by its density (g/mL). That is;Density = mass ÷ volume
Volume = mass ÷ density
The density of the blood sample is given as 1.05g/mL while the mass is given as 5.25g. The volume can be calculated as follows:Volume = 5.25 ÷ 1.05
Volume = 5mL
The The volume of the blood sample is 5mL.
Learn more: