Using the cell voltage measured for the first cell studied, with cell chemistry Zn/Zn^2+ \ Cu^2+/Cu, and the known half life potential for Zn^2/Zn calculate the reduction potential for Cu^2+/Cu and enter value below.
The information received for this problem were the values obtained during an online lab:
Cu xM cell voltage:1.100 V
Range: 0.005 V
Temp: 25 degrees C
Solved
Show answers
More tips
- F Food and Cooking Delicious and Simple Fillings for Pies...
- H Health and Medicine How to Help Men Gain Weight?...
- A Auto and Moto Discovering the Leader: What is the Most Expensive Car in the World?...
- H Health and Medicine Hangover: How to Get Rid of It Quickly?...
- S Style and Beauty How to Choose the Right Fur Coat and Avoid Regrets?...
- C Computers and Internet How to Create a Folder on Your iPhone?...
- G Goods and services How to sew a ribbon: Tips for beginners...
- F Food and Cooking How to Make Mayonnaise at Home? Secrets of Homemade Mayonnaise...
- C Computers and Internet Which Phone is Best for Internet Surfing?...
- F Food and Cooking Everything You Need to Know About Pasta...
Answers on questions: Chemistry
- C Chemistry Namespelling word meaningdartchargefortguardawardbackyardarguesparktargetmorningstorycarpetwarpdoorsmartstorkcordcoreborea. an analogy is a statement that compares sets...
- C Chemistry Beet juice is added to a unknown solution and changes color from red to purple. curry powder, added to the same solution, turns a deep red color. is the hydrogen concentration...
- C Chemistry 2points which of the following is a result of the specific heat differences between land and ocean?...
- C Chemistry Data table and reflection question : cup1(eggs +vinegar) day day 1 day 2 day 3 day4 cap2 (eggs +water)day1 day2 day3 day4 cap 3 (sugar cubes +vinegar)day1 day2 day 3 day4...
- C Chemistry Why are fruit juices stored in plastic bottles or paper carton not in a metal container?...
- C Chemistry Calculate the empirical formula of each of the following substances with the following compositions. a 3.60 g of magnesium and 10.65 g of chlorine b 9.1 g of lithium and...
- C Chemistry How many moles of carbon dioxide (CO2) does 250 grams of the substance have? How many molecules of CO2 are there? The molar mass of CO2 is 44.01 g/mol....
- C Chemistry The person who showed experimental evidence of electromagnetic waves and their link to light....
- C Chemistry Sodium bicarbonate (NaHCO3) is commercially known as baking soda. How many grams and atoms of oxygen are there in 15 grams of sodium bicarbonate? The molar mass of NaHCO3...
- C Chemistry What is the IUPAC name of the following compound?...

Ответ:
The reduction potential of is
is 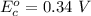
Explanation:
From the question we are told that
The potential of the cell is
The range is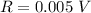
The temperature is
Note the reason why Zn is oxized and Cu is reduced is because Zn is higher than Cu on the electrochemical series
The reaction at the anode is
The
The reaction at the anode is
Now
substituting values
Hence the reduction potential of is
is 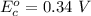
Ответ:
1) volumetric
2) graduated
3) volumetric
Explanation:
A volumetric glassware is a glassware that is marked at a particular point. A typical example of a volumetric glassware is the volumetric flask. A volumetric glassware is capable of measuring only a specific volume of a liquid.
On the other hand, graduated glassware can measure a range of volumes of liquid. However, a volumetric glassware is still required where a high degree of accuracy is important.