When 100.0 g block of a metal at 600 oC is plunged into 100.0 g of water (specific heat capacity 4.2 J/g.oC) at 30 oC, the final temperature of both the metal and the water is 80 oC. If no heat is lost to the surroundings, what is the specific heat capacity of the metal in J/g.oC? Give your answer to 2 significant figures.
Solved
Show answers
More tips
- H Health and Medicine What makes Graves’ disease dangerous?...
- W Work and Career 10 Best Ways To Find A Job: Tips To Land Your Dream Job...
- L Leisure and Entertainment How to Choose a Program for Cutting Music?...
- A Auto and Moto How to choose the right drive for your BMW...
- L Leisure and Entertainment How to Choose the Perfect Gift for Men on February 23rd?...
- H Health and Medicine How to Treat Whooping Cough in Children?...
- H Health and Medicine Simple Ways to Lower Cholesterol in the Blood: Tips and Tricks...
- O Other How to Choose the Best Answer to Your Question on The Grand Question ?...
- L Leisure and Entertainment History of International Women s Day: When Did the Celebration of March 8th Begin?...
- S Style and Beauty Intimate Haircut: The Reasons, Popularity, and Risks...
Answers on questions: Chemistry
- C Chemistry What is the element ar classified as in the periodic table?...
- C Chemistry Which element is more reactive? a) sulfur b) aluminum...
- C Chemistry Which of these statements about the organization of the periodic table is true? a. elements in the same column have the same number of valence electrons. b. elements near...
- C Chemistry What is conserved during a chemical reaction matter, energy, both, or niether?...
- C Chemistry Which type of atom has the strongest attraction for electrons in bond formation...
- C Chemistry Write a formula for the ionic compound that forms from each pair of elements. part a aluminum and oxygen...
- C Chemistry When chlorophyll is treated with acid, it loses its metal ion, producing the pigment pheophytin which is?...
- C Chemistry How would one describe the difference between saturated and unsaturated hydrocarbons and what is the distinguishing feature of aromatic hydrocarbons?...
- C Chemistry The Rydberg equation includes the math function with frequency proportional to 1/n2. If n is changed from 1 to 4, what happens to the frequency....
- C Chemistry what is the change in velocity of the cyclist below as he travels from point B to point C what is it his acceleration from point B to point C...

Ответ:
Explanation:
As we know that,
where,
q = heat absorbed or released
Now put all the given values in equation (1), we get
Therefore, the specific heat capacity of metal is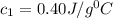
Ответ:
A: Central
B: Peripheral
Explanation: