jujulakaeuaws
13.09.2019 •
Chemistry
When 2.0 x 10-2 mole of nicotinic acid (amonoprotic
acid) is dissolved in 350 ml of water, the ph is 3.05.what is the
ka of nicotinic acid?
Solved
Show answers
More tips
- S Society and Politics Why are thugs called gopniks ? A fascinating journey through Russian subculture...
- A Animals and plants Want a Perfect Lawn? Learn How to Plant Grass the Right Way...
- A Animals and plants How to Properly Care for a Pet Decorative Rabbit at Home?...
- C Computers and Internet How to Check the Speed of My Internet?...
- H Health and Medicine 10 Ways to Cleanse Your Colon and Improve Your Health...
- W Work and Career How to Write a Resume That Catches the Employer s Attention?...
- C Computers and Internet Е-head: How it Simplifies Life for Users?...
- F Family and Home How to Choose the Best Diapers for Your Baby?...
- F Family and Home Parquet or laminate, which is better?...
- L Leisure and Entertainment How to Properly Wind Fishing Line onto a Reel?...
Answers on questions: Chemistry
- M Mathematics How do I simplify when there are fractions on fractions...
- P Physics Energy is the ability to do work. All of the following describe work being done EXCEPT: Select one: a. blowing out a candle s flame b. holding a heavy bowling ball c. lifting a mug...
- M Mathematics Solve for b. 39 = 28 - 6...

Ответ:
Ka of nicotinic acid =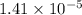
Explanation:
pH = 3.05
No. of mol of nicotinic acid =
Volume of water = 350 mL = 0.0350 L
Molarity =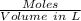
Molarity =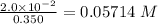
Nicotinic acid dissoctates as:
[H+] = 0.00089125 M
[A-] = 0.00089125 M
[HA} at equilibium = 0.05714 - 0.00089125 = 0.05624875 M
Ответ:
B. organ
Explanation:
Smooth muscle is found in the walls of hollow organs like your intestines and stomach. They work automatically without you being aware of them. Smooth muscles are involved in many 'housekeeping' functions of the body.