kenziedil2637
07.03.2020 •
Chemistry
Which of the following is true of gases?
A. Gases are not easily compressed because of expansion and limited space between particles.
B. Gases mix easily because of their high kinetic energy and low intermolecular forces.
C. Gas particles diffuse easily because of their low fluidity and high intermolecular forces.
D. Gas particles spread out to fill a container because of their high density and high kinetic energy.
Solved
Show answers
More tips
- F Food and Cooking Is Bacon Good for You?...
- S Style and Beauty Discover the Art of Nail Design: How Do You Paint Your Nails?...
- P Philosophy How to Develop Extrasensory Abilities?...
- O Other Everything You Need to Know About Kudyabliks...
- C Computers and Internet The Twitter Phenomenon: What it is and How to Use it...
- C Computers and Internet How to Choose a Laptop: Expert Guide and Tips...
- C Computers and Internet How to Choose a Monitor?...
- H Horoscopes, Magic, Divination Where Did Tarot Cards Come From?...
- S Style and Beauty How to Make Your Lips Fuller? Ideas and Tips for Beautiful Lips...
- C Computers and Internet How to Learn to Type Fast?...
Answers on questions: Chemistry
- E English I need help to complete the article. (English is not my first language)...
- B Business Live Trap Corporation received the data below for its rodent cage production unit. OUTPUT INPUT 50,200 cages Production time 625 labor hours Sales price: $3.60 per unit Wages $ 7.60...
- M Mathematics If three distinct numbers have a sum of 10 and a product of 20, what is their median...

Ответ:
B. Gases mix easily because of their high kinetic energy and low intermolecular forces
Explanation:
Gases have higher kinetic energy compared to solid and liquid because the large intermolecular spaces makes a large amount of movement between particles possible. The movements of the particles exhibits kinetic energy.
Gases are highly compressible because its particles aren’t tightly packed together. The intermolecular spaces are large and makes it easier for the gas particles to be compressed in a space which makes option A incorrect.
Gases have Low intermolecular molecules which explains why they aren’t tightly packed together which makes C incorrect.
Gases have a low density because the particles aren’t tightly packed together unlike the solids which are tightly packed together and denser than the liquid and gas which makes option D incorrect
Ответ:
Explanation:
Given parameters:
Mass of the sample = 0.622g
Volume of the gas = 2.4L
Temperature = 287K
Pressure of the gas = 0.85atm
Unknown
Molar mass of the gas = ?
Solution
To find the molar mass of the gas, we first assume that the gas in question is an ideal gas i.e. it obeys Boyle's and Charles's law.
On this premise, we can find the unknown which is the molar mass of the gas.
We first use the ideal gas equation to find the number of moles of the gas since all the parameters apart from the number of moles are given:Solving for number of moles:
The ideal gas equation is given as PV = nRT
We make the unknown "n" the subject of the equation
n =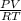
R is the gas constant whose value is 0.082atmdm³mol⁻¹K⁻¹
n =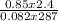
n =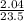 = 0.087mole
= 0.087mole
Now, we know the number of moles of the gas. We can proceed to find the molar mass from the given mass and the number of moles calculated using the equation below:molar mass =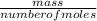
molar mass =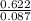 =7.15gmol⁻¹
=7.15gmol⁻¹
Part 2:
If the sample was placed under a low temperature, we want to consider what would happen to the volume:
Since we assume that the gas is an ideal gas, we can explain what will happen to the gas using Charles's law. The law states that "The volume of a fixed mass of a gas varies directly as its absolute temperature if the pressure is constant".
If we assume the pressure is constant for this gas, the volume of the gas would decrease as the temperature becomes lower. We would get lower values for both actual volume and predicted volume.