NayNay1105
06.08.2019 •
Chemistry
Which of the following statements is/are correct? 1. for a chemical system, if the reaction quotient (q) is greater than k, reactant must be converted to products to reach equilibrium.2. for a chemical system at equilibrium, the forward and reverse rates of reaction are equal.3. for a chemical system at equilibrium, the concentrations of products divided by the concentrations of reactants equals one.1 only2 only3 only1 and 21, 2, and 3
Solved
Show answers
More tips
- A Animals and plants Уход за джунгариками: полезные советы и рекомендации...
- L Legal consultation What Documents Are Required for a Russian Passport?...
- S Sport How to Choose Tennis Rackets?...
- H Health and Medicine AKDS Vaccination: Ensure Your Child s Safety...
- H Health and Medicine Naskol ko Opasen Ukus Kleshcha i Kak Ego Raspoznat...
- C Computers and Internet How to Delete Your Account on Odnoklassniki...
- H Health and Medicine What to Do When Your Jaw Locks Up?...
- G Goods and services What Are the Most Popular Services?...
- P Philosophy How did the concept of module arise in computer science?...
Answers on questions: Chemistry
- C Chemistry 1) your favorite recipe for preparing 100 brownies requires the following : 6 eggs , 5 cups flour and 2 sticks of butter . how many eggs would be required to yield 50 brownies...
- C Chemistry Because the the individual components of any mixture are not to each other, the composition of those components can vary. also, some of the properties of the individual components...
- C Chemistry How do people get minerals out of the ground?...
- C Chemistry Drag the tiles to the correct boxes to complete the pairs. Match each term with its description. niche commensalism parasitism mutualism a symbiotic interaction in which...
- C Chemistry Scientific knowledge is gained after debate and confirmation within the scientific community. Give an example when scientists debated over some new discovery, and finally...
- C Chemistry Refer to the following balanced equations 2GH14 + 190, 1200, 14H00 What mass of oxygen (Os) is required to react completely with 25.0 9 of C6H14?...
- C Chemistry Which answer would represent 0.001 moles?...
- C Chemistry How many moles of Neon gas are there if 25.0 Liters of the gas are at 278K and pressure of 89.9 KPa (R= 8.314)...
- C Chemistry Beryllium can be extracted from beryllium chloride. Beryllium chloride (BeCl2) is heated with potassium to produce be chloride (KCI). (d) Complete the equation for the reaction....
- C Chemistry A 1.00 liter solution contains 0.42 moles nitrous acid and 0.32 moles sodium nitrite . If 0.16 moles of nitric acid are added to this system, indicate whether the following...

Ответ:
Explanation:
A chemical equilibrium is defined as the state of reaction in which the rate of forward reaction is equal to the rate of backward reaction.
When Q >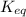 , then it means that the reaction is proceeding in the backward reaction. Whereas if Q <
, then it means that the reaction is proceeding in the backward reaction. Whereas if Q < 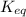 , then it means that the reaction is proceeding in the forward direction. Hence, formation of products will be favored.
, then it means that the reaction is proceeding in the forward direction. Hence, formation of products will be favored.
On the other hand, if Q =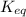 , then it means reaction is at equilibrium.
, then it means reaction is at equilibrium.
At equilibrium, it is not necessary that the concentrations of products divided by the concentrations of reactants equals one.
Thus, we can conclude that the statement for a chemical system at equilibrium, the forward and reverse rates of reaction are equal, is correct.
Ответ:
2 H2 + 1 O2 = 2 H2O
4 g H2 > 32 g O2 > 36 g H2O
↓ ↓ ↓
14.0 g > 2.0 g O2 > mass H2O ?
32 * mass H2O = 2.0 * 36
32 * mass H2O = 72
mass of H2O = 72 / 32
mass of H2O = 2.25 g
hope this helps!.