Which statement about the following reaction is correct?
ch4 (g) + 2o2 (g) yields co2 (g) + 2h2o(l) deltah = -890 kj
reacting one mole of oxygen (o2) absorbs 445 kj of energy
reacting one mole of oxygen (o2) releases 445 kj of energy
reacting one mole of methane (ch4) absorbs 890 kj of energy
reacting two moles of methane (ch4) releases 890 kj of energy
Solved
Show answers
More tips
Answers on questions: Chemistry
- C Chemistry Dos átomos con una diferencia de electronegatividad de 0.4 forman un enlace que es iónico, porque los electrones se transfieren covalente, porque los electrones se comparten iónico,...
- C Chemistry What specialized plant structures increase the probability of successful reproduction?...
- C Chemistry The coefficients in a balanced chemical equation represent...
- C Chemistry Would you expect to find older or newer fossils in rock layers closer to the surface? Why?...
- C Chemistry What is the concentration of a solution that has 87.6645 g of NaCl to make a 2.5 L solution? _ M a. 0.60 b. 1.7 c. 36 d. 5.6...
- C Chemistry 1. A mammal s teeth are adapted to its diet. Some mammals of their teeth have flat surfaces that enable the animals to crush and grind the tough material in plant parts. Which...
- C Chemistry From the list provided, choose the reason that best matches your reasoning. The triple beam balance is for science classrooms and is more accurate. The kitchen scale measures...
- C Chemistry Can someone help with either example 3 and 4 please Im still learning how to do all this and it’s very hard for me...
- C Chemistry The use of genetics by farmers to produce more abundant and healthier crops is an application of the science of . DNA profiling biotechnology molecular biology nuclear genetics...
- C Chemistry how could you check that when the calcium carbonate is reacting with hydrochloric acid the gas given off is carbon dioxide...

Ответ:
reacting one mole of oxygen (O2) releases 445 kJ of energy
Explanation: Exothermic reactions are defined as the reactions in which energy is released in the form of heat and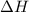 for the reaction comes out to be negative.
for the reaction comes out to be negative.
According to the given balanced equation:
1 mole of methane reacts with 2 moles of oxygen and 890 kJ of energy is released.
Thus when the reaction is halved, the enthalpy also gets halved.
Thus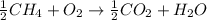

Now half mole of methane reacts with 1 mole of oxygen and 445 kJ of energy is released.
Ответ:
first answer is 12x and the seconds answer is: they are both in simplest form buy one of em are in radical form and the other is in simplest form
Step-by-step explanation:
im sorryyy if it's wrong