carlosiscr7
09.10.2021 •
Chemistry
Write a balanced net ionic equation to show why the solubility of ZnCO3 (s) increases in the presence of a strong acid and calculate the equilibrium constant for the reaction of this sparingly soluble salt with acid.Consider only the FIRST STEP in the reaction with strong acid. Be sure to specify states such as (aq) or (s).
Solved
Show answers
More tips
- F Food and Cooking How to Cook a Steak with Mustard Sauce? What Kind of Meat to Choose?...
- P Photography and Videography What is lens calibration and why is it needed?...
- F Family and Home Stay Warm but Don t Overheat: What is the Optimal Temperature for Your Home During Winter?...
- H Health and Medicine How to Treat the Flu: A Comprehensive Guide...
- O Other What is a Disk Emulsifier and How Does it Work?...
- F Family and Home What does a newborn need?...
- F Family and Home Choosing the Right Car Seat for Your Child: Tips and Recommendations...
- F Food and Cooking How to Get Reconfirmation of Registration?...
- C Computers and Internet How to Get Rid of Spam in ICQ?...
- A Art and Culture Who Said The Less We Love a Woman, the More She Likes Us ?...
Answers on questions: Chemistry
- M Mathematics Help with statistics Width Lower class limit Upper class limit...
- M Mathematics Apair of equations is shown below: y = 3x − 5 y = 6x − 8 part a: explain how you will solve the pair of equations by substitution or elimination. show all the steps and write...
- M Mathematics Create a navigation question using this triangle....
- B Business What do people purchase as a form of risk management to protect themselves from losing a lot of money in the event something happens to them or their property? 1. Deductibles...

Ответ:
Answer : The mass of oxygen required are, 56.448 grams
Explanation : Given,
Mass of glucose = 53 g
Molar mass of glucose = 180.156 g/mole
Molar mass of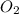 = 32 g/mole
= 32 g/mole
First we have to calculate the moles of .
.
Now we have to calculate the moles of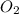 .
.
The balanced chemical reaction will be,
From the balanced chemical reaction, we conclude that
As, 1 mole of react with 6 moles of
react with 6 moles of 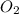
So, 0.294 mole of react with
react with 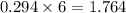 mole of
mole of 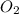
Now we have to calculate the mass of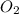 .
.
Therefore, the mass of oxygen required are, 56.448 grams