zanaplen27
01.07.2020 •
Physics
A 12 cm diameter piston-cylinder device contains air at a pressure of 100 kPa at 24oC. The piston is initially 20 cm from the base of the cylinder. The gas is now compressed and 0.1 kJ of boundary work is added to the gas. The temperature of the gas remains constant during this process.
a. How much heat was transferred to/from the gas?
b. What is the final volume and pressure in the cylinder?
c. Find the change in entropy of the gas. Why is this value negative if entropy always increases in actual processes?
Solved
Show answers
More tips
- F Family and Home How to Raise a Genius? Discover the Secrets Here...
- F Food and Cooking How to Properly Cook Buckwheat?...
- H Health and Medicine How to Tan in a Tanning Bed? Tips and Recommendations...
- S Style and Beauty How to Sew a Balloon Skirt: Detailed Tutorial and Tips on Choosing the Right Fabric...
- P Philosophy Personal attitude towards Confession: how to prepare and undergo the procedure correctly?...
- F Food and Cooking How to Make Cottage Cheese at Home: Simple and Quick Recipe with Step By Step Instructions...
- H Health and Medicine Which Water Are You Drinking? Is it Worth Buying Bottled Water?...
- H Health and Medicine What Makes a Man a Man?...
- H Health and Medicine What to Take with You to the Maternity Hospital?...
- F Food and Cooking What Foods Can Nursing Moms Eat?...
Answers on questions: Physics
- P Physics Prove that horizontal range of a projectile is same, when Ered at an angle 8 and 90° -6) with the horizontal....
- P Physics An airplane is flying on a course of 45° at 400 mph. A 70 mph wind coming from the east.What is the speed of the plane? A. 354 mi/hr B. 452 mi/hr C. 400 mi/hr...
- P Physics A blue textbook will green light. A) emit B) reflect C) absorb D) transmit...
- P Physics Which of the following is a source of visible light? A) a cat B) a sofa C) a lamp D) a plant...
- P Physics Describe what happens to the system inside of a refrigerator or freezer in terms of heat transfer, work, and conservation of energy. Confine yourself to time periods in which the door...
- P Physics You may have noticed that antennas are mounted vertically on cars, pretty much universally. What interaction (and what component) of the radio wave and the antenna orientation explains...
- M Mathematics Bowls are purchased for $14 each and sold for $35.00. what is the percent of the markup based on cost?...
- M Mathematics Is the following always, sometimes, or never true? 14+3x-7=7x+7-4x a.always b.sometimes c.never...
- M Mathematics Bailey got 7 bows for her birthday.at school,she lost a third of her bows.when she got home,she realized she had 42 bows left.how many bows did she have before her birthday? -...
- E English You can check out if mi answers is correct.....

Ответ:
ΔQ = 0.1 kJ
ΔS = -0.337 J/K
The value negative is due to the fact that there is need to be the same amount of positive change in surrounding as a result of compression.
Explanation:
GIven that:
Diameter of the piston-cylinder = 12 cm
Pressure of the piston-cylinder = 100 kPa
Temperature =24 °C
Length of the piston = 20 cm
Boundary work ΔW = 0.1 kJ
The gas is compressed and The temperature of the gas remains constant during this process.
We are to find ;
a. How much heat was transferred to/from the gas?
According to the first law of thermodynamics ;
ΔQ = ΔU + ΔW
Given that the temperature of the gas remains constant during this process; the isothermal process at this condition ΔU = 0.
Now
ΔQ = ΔU + ΔW
ΔQ = 0 + 0.1 kJ
ΔQ = 0.1 kJ
Thus; the amount of heat that was transferred to/from the gas is : 0.1 kJ
b. What is the final volume and pressure in the cylinder?
In an isothermal process;
Workdone W =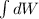
Since the gas is compressed ; then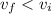
However;
The initial volume for the cylinder is calculated as ;
Replacing over values into the above equation; we have :
The final pressure can be calculated by using :
c. Find the change in entropy of the gas. Why is this value negative if entropy always increases in actual processes?
The change in entropy of the gas is given by the formula:
where
T = 24 °C = (24+273)K
T = 297 K
ΔS = -0.337 J/K
The value negative is due to the fact that there is need to be the same amount of positive change in surrounding as a result of compression.
Ответ:
second one
Explanation: