mariahcrook7
03.01.2020 •
Physics
How much heat energy is required to convert 93.4 g of solid ethanol at − 114.5 ° c to gasesous ethanol at 149.8 ° c ? the molar heat of fusion of ethanol is 4.60 kj/mol , and its molar heat of vaporization is 38.56 kj/mol . ethanol has a normal melting point of − 114.5 ° c and a normal boiling point of 78.4 ° c . the specific heat capacity of liquid ethanol is 2.45 j / g ⋅ ° c , and that of gaseous ethanol is 1.43 j / g ⋅ ° c .
Solved
Show answers
More tips
- F Food and Cooking Delight for Gourmets: How to Prepare Liver Pate...
- S Style and Beauty How to braid friendship bracelets?...
- H Health and Medicine Mercury Thermometer Danger: What to do when a thermometer breaks?...
- F Food and Cooking Which Calamari Salad is the Most Delicious?...
- S Society and Politics 10 Tips for Boosting Your Self-Esteem...
- F Food and Cooking The Most Delicious and Simple Fish in Batter Recipe...
- H Health and Medicine What is Autism? Understanding the Basics of This Neurodevelopmental Disorder...
- P Philosophy How to Develop Extrasensory Abilities?...
- S Style and Beauty Don t Sacrifice Your Brows: How to Properly Pluck Stubborn Hairs...
- W Work and Career 10 Best Ways To Find A Job: Tips To Land Your Dream Job...
Answers on questions: Physics
- S Social Studies Dr. hendricks, a psychologist, believes that the differences between the genders can be attributed to the varying levels of estrogen and testosterone in males and...
- E English Fast with these two questions. 1. when my mother caught me out past my curfew, the look in her eyes _ a very bad punishment a. immolated b. quelled c. portended...
- B Biology The control center and director of cell activities is the endoplasmic reticulum nucleus mitochondria lysosomes...
- M Mathematics I am not very good at math, and my daughter finished her math assignment and wasn t sure if she did everything correctly. Can someone make sure this is correct?...
- M Mathematics Consider the quadratic function: f(x) = x2 – 8x – 9 Vertex:...

Ответ:
Q' = 140.859 kJ
Explanation:
Given that, 93.4 g of solid ethanol at − 114.5 °C is converted to gasesous ethanol at 149.8 ° C.
The molar heat of fusion of ethanol is, ΔH(f) = 4.60 kJ/mol , and its molar heat of vaporization is ΔH(v) = 38.56 kJ/mol .
And also Ethanol has a normal melting point of − 114.5 ° C and a normal boiling point of 78.4 ° C .
The specific heat capacity of liquid ethanol is S(l) = 2.45 J / g ⋅°C , and that of gaseous ethanol is S(g) = 1.43 J / g ⋅°C .
Lets solve this step wise ;
Given 93.4 g of ethanol is taken, but 1 mole of ethanol consists of 46.06 g
⇒ moles of ethanol given =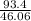 = 2.02 moles
= 2.02 moles
step 1: solid ethanol to liquid ethanol at melting point of − 114.5 ° C
⇒ 1 mole requires ΔH(f) = 4.60 kJ/mol of heat
⇒ heat required = 4.60 × 2.02 = 9.292 kJ.
step 2: liquid ethanol at -114 °C to liquid ethanol at 78.4 °C
Q = m×S×ΔT ; Q = heat required
m = mass of the substance
S = specific heat of the substance
ΔT = change in temperature
Here S = S(l);
⇒ Q = 93.4×2.45×(78.4-(-114.5))
= 44.141 kJ
step 3: liquid ethanol at 78.4°C to gaseous ethanol at 78.4°C
1 mol of liquid ethanol requires ΔH(v) = 38.56 kJ/mol of heat
⇒ required heat = 38.56×2.02 = 77.89 kJ
step 4: gaseous ethanol at 78.4 °C to gaseous ethanol at 149.8 °C
Q = m×S×ΔT
Here, S = S(g)
Q = 93.4×1.43×(149.8-78.4)
= 9.536 kJ
⇒ Total heat required = 9.292 + 44.141 + 77.89 + 9.536
= 140.859 kJ
⇒ Q' = 140.859 kJ
Ответ:
The heat loss per unit length is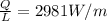
Explanation:
From the question we are told that
The outer diameter of the pipe is
The thickness is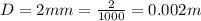
The temperature of water is
The outside air temperature is
The water side heat transfer coefficient is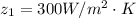
The heat transfer coefficient is
The heat lost per unit length is mathematically represented as
Substituting values