linhlema2005
02.03.2020 •
Chemistry
A 11.6 g piece of metal is heated to 98°C and dropped into a calorimeter containing 50.0 g of water (specific heat capacity of water is 4.18 J/g°C) initially at 20.5°C. The empty calorimeter has a heat capacity of 125 J/K. The final temperature of the water is 28.2°C. Ignoring significant figures, calculate the specific heat of the metal.
Solved
Show answers
More tips
- S Sport Running: How to Do It Right?...
- F Food and Cooking How to Cook Spaghetti Right – Secrets and Tips...
- P Philosophy Personal attitude towards Confession: how to prepare and undergo the procedure correctly?...
- H Health and Medicine Flu: How to Recognize It by the First Symptoms?...
- F Food and Cooking How to Sober Up Quickly? Important Facts and Tips...
- H Health and Medicine How to Properly Take a Blood Sugar Test?...
- H Health and Medicine Simple and Effective: How to Get Rid of Cracked Heels...
- O Other How to Choose the Best Answer to Your Question on The Grand Question ?...
- L Leisure and Entertainment History of International Women s Day: When Did the Celebration of March 8th Begin?...
- S Style and Beauty Intimate Haircut: The Reasons, Popularity, and Risks...
Answers on questions: Chemistry
- C Chemistry Which graph best matches a person walking away slowly and returning quickly...
- M Mathematics In the Dallas Cowboys stadium, which has an open roof, 33% of the seats are in the shade for most of the game, while rest of the seats are in the sun. The stadium has 80,815 seats....
- B Biology What are the properties of water that make it necessary for life?...
- M Mathematics This year, students in the 9th grade are collecting dimes and quarters for a school fundraiser. They are trying to collect more money than the students who were in the 9th grade...
- H History Identify Jefferson s overall view of the Federal Government- * A. The Federal government should protect the country and leave the state governments to make laws governing themselves...
- H History Why did Henry VIII join the protestant Reformation?...

Ответ:
Answer : The specific heat of the metal is,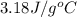
Explanation :
Heat released by the reaction = Heat absorbed by the calorimeter + Heat absorbed by the water
where,
q = heat released by the reaction
c = specific heat of metal = ?
m = mass of metal = 11.6 g
Now put all the given values in the above formula, we get:
Thus, the specific heat of the metal is,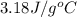
Ответ:
common glass
Explanation:
the test