QueenNerdy889
21.10.2020 •
Chemistry
A 32.149 mg sample of a chemical known to contain only carbon hydrogen sulfur and oxygen is put into a combustion analysis apparatus yielding 57.271 mg of co2 and 23.444 mg of h2o. In another experiment 22.345 mg of the compound is reacted with excess oxygen to produce 9.656 mg of sulfur dioxide
Solved
Show answers
More tips
- C Computers and Internet Make Easy Accessible Screenshots on iPad in Just a Few Minutes...
- H Health and Medicine Heartburn: Causes and Ways to Get Rid of It...
- H Health and Medicine Simple and Effective: How to Get Rid of Cracked Heels...
- H Health and Medicine Relieving Swelling in Legs: Causes and Ways to Alleviate the Symptom...
- W Work and Career Мерчендайзинг – все, что нужно знать...
- O Other Everything You Need to Know About Kudyabliks...
- F Food and Cooking How to cook crayfish? Everything you need to know...
- F Food and Cooking Homemade kvass: recipe and brewing process...
- H Health and Medicine How to Choose the Right Tanning Cream?...
- S Style and Beauty Secrets of Tying a Pareo: 5 Ways...
Answers on questions: Chemistry
- C Chemistry What does the presence of a polar covalent bond show about the electronegativity of its two atoms...
- C Chemistry Gases ___ out and fill their container completely...
- C Chemistry HELP PLEASE! The production of carbon-14… a. occurs to a large extent in nuclear reactors b. is mostly due to fallout from nuclear explosions c. takes place in the upper atmosphere...
- C Chemistry There are two designs for roller coasters awaiting approval at Disneyland Headquarters in Florida. Both designs use 3000 cm of track and have similar shapes and heights. In...
- C Chemistry PLEASE HELP ON EASY CHEMICAL PROPERTIES...
- C Chemistry 3. If you take a sample of gaseous water and continually compress it, what will happen to the particles? What, if any, phase changes will occur?...
- C Chemistry Moving from the element with atomic number 10 to atomic number 11 on the periodic table, there is a change in reactivity. In three to five sentences, identify the direction...
- C Chemistry Starches and sugars being broken down during energy production a Chemical Change b Physical Change...
- C Chemistry cars are bumpers to bumper traveling at a speed of 45 miles/hr. in two days, how many cars would pass a specific point? each car is 18 ft long?...
- C Chemistry Evaporation of water off of the surface of leaves a Chemical Change b Physical Change...

Ответ:
C₅H₁₀SO₂
Explanation:
From the given information:
The objective is to identify the empirical formula of the compound.
To start with determining the mass of carbon from carbon dioxide; we have:
Mass of carbon = 12/44 × 57.271 mg
Mass of carbon = 0.2727 × 57.271 mg
Mass of carbon = 15.6178 mg
The mass of hydrogen is:
Mass of hydrogen = 2.6257 mg
The mass of sulphur is:
Mass of Sulphur =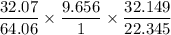
Mass of Sulphur = 6.9550 mg
The Mass of oxygen can now be = mass of (Sample - Carbon - Hydrogen - Sulphur)
= (32.149 - 15.6178 - 2.6257 - 6.9550)g
= 6.9505 g
Recall that:
number of moles = mass/molar mass
Thus:
The moles of C : H : S : O are:
= 1 : 2 : 0.2 : 0.4
Divide by the smallest; we have:
= 1/0.2 : 2/0.2 : 0.2/0.2 : 0.4/0.2
= 5 : 10 : 1 : 2
Thus, the empirical formula is = C₅H₁₀SO₂
Ответ:
the answer should be 22.4L