buchaell000
07.06.2020 •
Chemistry
A sample tube consisted of atomic hydrogen in their ground state. A student illuminated the atoms with monochromatic light, that is, light of a single wavelength. If only two separate emission lines in the visible region are observed, what is the wavelength (or wavelengths) of the incident radiation?
Solved
Show answers
More tips
- O Other What is the oldest joke ever told?...
- F Food and Cooking How to Make Lazy Cabbage Rolls? Simple Steps to a Delicious Dish...
- F Food and Cooking Unusually Delicious Shashlik - Follow the Etiquette of Proper Preparation!...
- L Leisure and Entertainment Couchsurfing: A New Way to Travel...
- G Goods and services Which TV is better - LCD or Plasma?...
- S Sport How to Learn to Pull Up on Monkey Bars?...
- L Leisure and Entertainment Scrapbooking: What is it and Why is it Becoming More Popular?...
- C Computers and Internet Where did torrents.ru move to?...
- B Business and Finance Understanding Cash Flow: What It Is and How It Works...
- C Computers and Internet What Are Peers and Seeds in Torrenting?...
Answers on questions: Chemistry
- C Chemistry Your Turn! How many electrons are required to complete the octet around nitrogen, when it forms N2 ? A. 2 B. 3 C. 1 D. 4 E. 6...
- C Chemistry The genetic code from DNA is carried into the cytoplasm by...
- C Chemistry Which property do metalloids share with nonmetals? Both are brittle. Both are shiny. Both have low melting points. Both are very conductive.\...
- C Chemistry A student collected 7.46 moles of I2 gas at STP How many liters is that?...
- C Chemistry Y Consider the graph of the line y = 1/2 x - 4 and the point (-4,2). The slope of a line parallel to the given line is 8 A point on the line parallel to the given line,...
- C Chemistry What the atomic number what is the mass number #protons ? and neutrons and electrons?...
- C Chemistry Help An igneous rock has small, dark crystals. Which statement also accurately describes this rock? It is an intrusive rock. It has a coarse texture. It was found on Earth’s...
- C Chemistry A student adds a 3.0 g antacid tablet to 35.0 g of water in a bottle. After the reaction is complete, 37.3 g of liquid remains in the bottle. How much gas was formed in...
- C Chemistry What is a State I need the definition...
- C Chemistry The actual yield of a product in a reaction was measured as 4.20g.if the theoretical yield of the product for the reaction is 4.88g,what is the percentage yield of the product...

Ответ:
The wavelength of the monochromatic light is 486.2 nm.
Explanation:
The illumination of the hydrogen atom by the monochromatic light causes an absorption of energy by its electrons which causes an excitation. After a period, the particle de-excites (decays) losing the absorbed energy and falls back to its initial state releasing the energy in the form of a photon. This photon can be observed as a colored light of the Balmer series.
From Rydberg's expression,
1/λ=−R( −
−  )
)
The transition of the electron is from n = 2 to 4, so that;
1/λ = R ( -
-  )
)
= 1.097 x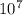 (
( -
-  )
)
1/λ = 2056875
So that,
λ =
= 4.8617 x m
m
The wavelength of the monochromatic light is 486.2 nm.
Ответ:
These organelles are like the organs in a human and they help the cell stay alive. Each organelle has it's own specific function to help the cell survive. The nucleus of a eukaryotic cell directs the cell's activities and stores DNA. ... All of the cell's organelles must work together to keep the cell healthy.
Hope this helps!
Janicey
(If you find this answer very helpful be sure to mark me brainliest!