aiselsarmientop8o290
02.12.2019 •
Chemistry
At 25°c, the standard enthalpy of combustion of gaseous propane (c3h8) is –2219.0 kj per mole of propane, and the standard enthalpy of combustion of gaseous propylene (c3h6) is –2058.3 kj per mole of propylene.
what is the standard enthalpy change for the following reaction at 25°c? c3h6(g) + h2(g) → c3h8(g)substance∆h°f (kj/mol)co2(g)–393.5h2o(l)–285.8
Solved
Show answers
More tips
- C Computers and Internet 3D Glasses! What is this thing?...
- C Computers and Internet How to insert videos into LiveJournal?...
- C Computers and Internet How Much Does an iPhone Cost in America?...
- F Family and Home How to Choose the Best Diapers for Your Baby?...
- F Family and Home Parquet or laminate, which is better?...
- L Leisure and Entertainment How to Properly Wind Fishing Line onto a Reel?...
- L Leisure and Entertainment How to Make a Paper Boat in Simple Steps...
- T Travel and tourism Maldives Adventures: What is the Best Season to Visit the Luxurious Beaches?...
- H Health and Medicine Kinesiology: What is it and How Does it Work?...
- O Other How to Choose the Best Answer to Your Question on The Grand Question ?...
Answers on questions: Chemistry
- C Chemistry 6.67*10^-11 * 75 * 6.4 * 10^23 / (85 * 10^6)^2mass of the moomn...
- C Chemistry Can i be someones gay friend? i prefer it if you were a girl cause if your a guy ill try to hit on you...
- C Chemistry What cultural differences does herodotus notice between greek ways of living and those of persia and egypt...
- C Chemistry Shaun is giving a presentation on how to use a certain technique in oil painting. For his presentation, he wants to use a friend s video that demonstrates the technique....
- C Chemistry Describe how a magnesium ion and an astatine ion are formed from a magnesium atom and an astatine atom . Will GIVE BRAINLIEST (if correct )...
- C Chemistry PLEASE HELP FOR EXAM I ll give 5 star and brainliest calculate the new volume if you have 2.00 L of a gas at 0 degrees Celsius, and raise the temp to 23 degrees Celsius....
- C Chemistry Which solution will boil at the highest temperature? * 1 mole of sugar in 500 g of water 1 mole of sugar in 1,000 g of water 2 moles of sugar in 500 g of water 2 moles...
- C Chemistry Necesito ayuda porfavor...
- C Chemistry Which element does not have ability to form an ionic or covalent bond...
- C Chemistry Which of the following is an example of a model? a-pv b-a geographical map c- a belief that aliens exist d-all the above...

Ответ:
The standard enthalpy change for the given reaction is 868.05 kJ
Explanation:
Calculating the enthalpy of propane:The chemical equation for the combustion of propane follows:
The equation for the enthalpy change of the above reaction is:
We are given:
Putting values in above equation, we get:
The enthalpy of formation of is -140.7 kJ/mol
is -140.7 kJ/mol
Calculating the enthalpy of propylene:The chemical equation for the combustion of propane follows:
The equation for the enthalpy change of the above reaction is:
We are given:
Putting values in above equation, we get:
The enthalpy of formation of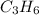 is -1008.75 kJ/mol
is -1008.75 kJ/mol
Calculating the enthalpy change of the reaction:The given chemical equation follows:
The equation for the enthalpy change of the above reaction is:
We are given:
Putting values in above equation, we get:
Hence, the standard enthalpy change for the given reaction is 868.05 kJ
Ответ:
Explanation:
... to Find E° for a New Half-Reaction ... Polarographic Measurement of an Equilibrium Constant. 19. ... present Chemical Demonstrations, but I can tell you about some of my favorites and show ... Significant figures are discussed in Chapter 3. ... Analysis of Unknown Calcium in a 5.00-mL urine sample was precipitated with.