datstepparoddoe27
11.12.2019 •
Chemistry
Calculate δhrxn for the following reaction: ch4(g)+2cl2(g)→ch2cl2(g)+2hcl(g) use the following reactions and given δh values. ch4(g)+cl2(g)→ch3cl(g)+hcl(g), δh=−99.60 kj ch3cl(g)+cl2(g)→ch2cl2(g)+hcl(g), δh=−105.8 kj express your answer to four significant figures.
Solved
Show answers
More tips
- S Style and Beauty Why is Sugaring Better than Waxing for Hair Removal?...
- W Work and Career Where can you learn to be a flight attendant?...
- G Goods and services How to Properly Calculate the Power of Your Air Conditioner?...
- F Food and Cooking Effective Methods to Organize Videos in your iPad According to Content...
- F Family and Home Parquet or laminate, which is better?...
- L Leisure and Entertainment How to Properly Wind Fishing Line onto a Reel?...
- L Leisure and Entertainment How to Make a Paper Boat in Simple Steps...
- T Travel and tourism Maldives Adventures: What is the Best Season to Visit the Luxurious Beaches?...
- H Health and Medicine Kinesiology: What is it and How Does it Work?...
- O Other How to Choose the Best Answer to Your Question on The Grand Question ?...
Answers on questions: Chemistry
- C Chemistry When suspended energy converts to energy in motion, we call this conversion . Energy stored in bonds between atoms is called potential energy. While in storage, the energy is potential...
- C Chemistry Why does metal expand when heated?...
- C Chemistry 1. How old was Beah, when the war broke out in his country? 2. How did Beah end up as a solder? 3. How did war change lives in Sierra Leone?...
- C Chemistry Plz hurry you don t h...
- C Chemistry Rank the crystal lattice structures in order of decreasing efficiency of space in the structure....
- M Mathematics Which of the following numbers has the largest absolute value? a) 16 b) 12.1 c) -2 d) -15...
- H History Please help!! Which of the following completes the organizer above? a. Arizona b. Washington c. Florida d. Oregon...
- M Mathematics Which of the following ordered pairs represents the unit rate?...
- E English What are some challenges in identifying what motivates others? What happens if our inferences about others motivations are incorrect ?...
- C Chemistry The periodic table is organized left to right by . Metals, nonmetals, metalloids Metals, metalloids, nonmetals Metalloids, nonmetals, metals Nonmetals, metalloids, metals...

Ответ:
The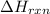 for the reaction is -205.4 kJ.
for the reaction is -205.4 kJ.
Explanation:
Hess’s law of constant heat summation states that the amount of heat absorbed or evolved in a given chemical equation remains the same whether the process occurs in one step or several steps.
According to this law, the chemical equation is treated as ordinary algebraic expressions and can be added or subtracted to yield the required equation. This means that the enthalpy change of the overall reaction is equal to the sum of the enthalpy changes of the intermediate reactions.
The chemical equation for the reaction of methane and chlorine gas follows:
The intermediate balanced chemical reaction are:
(1)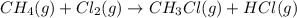
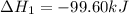
(2)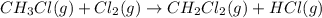
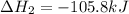
The expression for enthalpy of the reaction follows:
Putting values in above equation, we get:
Hence, the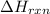 for the reaction is -205.4 kJ.
for the reaction is -205.4 kJ.
Ответ:
Part 1: x ≥ 17/9
Part 2: x is greater than or equal to seventeen ninths.
Part 3: using x= 3 and 4 respectively, the solution was verified
The complete statement related to this found on (ID: 2020133) is stated below:
Use the inequality to answer Parts 1-3.
-3(x - 2) ≤ one third
Part 1: Solve the inequality. Leave answer in terms of a whole number or reduced improper fraction.
Part 2: Write a verbal statement describing the solution to the inequality.
Part 3: Verify your solution to the inequality using two elements of the solution set.
Use a word processing program or handwrite your responses to Parts 1-3. Turn in all three responses.
Step-by-step explanation:
Part 1) The inequality: -3(x - 2) ≤ one third
-3(x - 2) ≤ ⅓
Multiply through by 3
-3(3)(x-2) ≤ 3(⅓)
-9(x-2) ≤ 1
Expand the bracket
-9x + 18 ≤ 1
Collect like terms
-9x ≤ 1-18
-9x ≤ -17
Divide through by coefficient of x (-9)
-9x/-9 ≤ -17/-9
When you divide an expression in inequality by a negative sign, the inequality sign changes
x ≥ 17/9
Part 2) the verbal statement of the solution:
x is greater than or equal to seventeen ninths.
Part 3) We would verify the solution to the inequality using two elements of the solution set greater than 17/9.
17/9 = 1.89
Let's use x= 3 and x = 4. As they are greater than 17/9
-3(x - 2) ≤ ⅓
When x= 3
-3(3 - 2) ≤ ⅓
-3(1) ≤ ⅓
-3 ≤ ⅓
This satisfies the solution of the inequality
When x= 4
-3(4 - 2) ≤ ⅓
-3(2) ≤ ⅓
-6 ≤ ⅓
This satisfies the solution of the inequality