wolverine123
19.08.2021 •
Chemistry
Choose the best possible answer for Q.No. A, B, and C.
The following is an acid-base reaction between sulfuric acid and aluminum hydroxide. Use the provided molar masses to answer the questions below:
molar
mass
3 H2SO4
+
2 Al(OH)3
⟶
Al2(SO4)3
+
6 H2O
g/mol
98.08
78.00
342.15
18.02
Note: You need to upload the documents for A, B, and C to show your work.
A. How many moles of sulfuric acid would be required to form 65.21g of Water?
moles of H2SO4 =
[ Select ]
B. How many grams of Aluminum Sulfate could be formed if 197.0g aluminum hydroxide reacts with sulfuric acid?
mass of Al2(SO4)3 =
[ Select ]
C. To determine the concentration of a sulfuric acid sample, a titration is performed with the above reaction. What is the concentration of the sulfuric acid if a 15.00mL sample reached its titration endpoint with 29.26mL of 0.2855M Al(OH)3?
Molarity of H2SO4 =
[ Select ]
Solved
Show answers
More tips
- F Food and Cooking How to Cook Julienne? Recipes and Tips...
- D Dating, Love, Relationships 10 Useful Tips on How to Survive a Breakup?...
- F Food and Cooking Apple Cider Vinegar: The Ultimate Health and Beauty Solution...
- C Computers and Internet Е-head: How it Simplifies Life for Users?...
- F Family and Home How to Choose the Best Diapers for Your Baby?...
- F Family and Home Parquet or laminate, which is better?...
- L Leisure and Entertainment How to Properly Wind Fishing Line onto a Reel?...
- L Leisure and Entertainment How to Make a Paper Boat in Simple Steps...
- T Travel and tourism Maldives Adventures: What is the Best Season to Visit the Luxurious Beaches?...
- H Health and Medicine Kinesiology: What is it and How Does it Work?...

Ответ:
c i think
Explanation:
Ответ:
The reaction of propylamine with water gives a hydrogen shift from water molecule to amine group of propylamine.
Further explanation:
Acids and bases can be defined in many ways based on different theories, which are as follows:
According to Arrhenius theory, acid is defined as the one which produces hydrogen ions in a solution, while the base is defined as the one which produces hydroxide ions in a solution. According to Bronsted–Lowry theory, the acid in the reaction donates a proton while a base is one that accepts a proton. According to Lewis theory, the acid in the reaction accepts a pair of electrons while a base donates a pair of electrons.The propylamine contains amine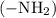 group. Amine
group. Amine 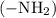 group is a basic group that can accept a proton from proton donor species because of the lone pair of nitrogen.
group is a basic group that can accept a proton from proton donor species because of the lone pair of nitrogen.
The water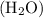 molecule has acidic hydrogen and thus water can behave as a proton donor molecule.
molecule has acidic hydrogen and thus water can behave as a proton donor molecule.
The water will release the proton to form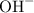 and propylamine will accept the proton to form
and propylamine will accept the proton to form 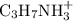 .
.
The complete reaction of propylamine with water gives a hydrogen shift from water molecule to amine group of propylamine as follows:
Learn more:
1. Salts produced from neutralization reaction:
2. Determine the reason that explains salt produce colored flame?:
Answer details:
Grade: Senior school
Subject: Chemistry
Chapter: Acids and bases
Keywords: Acids, bases, nh2, h2o, bronsted–Lowry theory, proton acceptor, proton donor, propylamine, reaction.