TerronRice
07.03.2020 •
Chemistry
Coal can be used to generate hydrogen gas (a potential fuel) by thefollowing endothermic reaction.C(s) + H2O (g) <==> CO(g) + H2(g)if this reaction mixture is at equilibrium, predict whether each ofthe following will result in the formation of additional hydrogengas, the formation of less hydrogen gas, or have no effect on thequantity og hydrogen gas.a. adding more C to the reaction mixtureb. adding more H2O to the reaction mixturec. raising the temperature of the reaction mixtured. increasing the volume of the reaction mixturee. adding a catalyst to the reaction mixturef. adding an inert gas to the reaction mixture
Solved
Show answers
More tips
- L Leisure and Entertainment Scrapbooking: What is it and Why is it Becoming More Popular?...
- H Horoscopes, Magic, Divination How to Cast a Love Spell on a Guy? Guide for Guys...
- F Family and Home How to Sew Curtain Tapes: Best Tips from Professionals...
- S Style and Beauty How are artificial nails removed?...
- S Style and Beauty How to Make Your Lips Fuller? Ideas and Tips for Beautiful Lips...
- F Food and Cooking The Disease That Haunted Abraham Lincoln...
- C Computers and Internet How to Get Rid of Windows Genuine Check?...
- H Health and Medicine How to perform artificial respiration?...
- S Style and Beauty Tricks and Tips: How to Get Rid of Freckles...
- C Computers and Internet How Do You Refill Cartridges?...
Answers on questions: Chemistry
- C Chemistry A plane stops from 500 miles per hour in 40 seconds. What was the planes acceleration? -460 mph/s -12.5 mph/second .085 mph/s 545 mph/second...
- C Chemistry 4. What is the dependent variable in the statement below? If the amount of coffee that Megan drinks before bed increases, then the amount of sleep she gets will decrease....
- C Chemistry You are less likely to see a total solar eclipse than a total lunar eclipse because a. the moon’s shadow covers all of earth during a solar eclipse. b. new moon phases...
- C Chemistry What is the molarity (m) of a solution that contains .25 moles of a compound in 1.8 l of solution?...
- C Chemistry Why is cooking an endothermic process?...
- C Chemistry Acertain chemical reaction releases 16.8/kjg of heat for each gram of reactant consumed. how can you calculate what mass of reactant will produce 800.j of heat? set...
- B Business In the past, the majority of all investment funds in China were channeled through the state banks but, this model is changing,...
- A Arts Can u guys help me finish the sentence for my homework we don t need permission to A.dynamite B. butter C. dance...
- M Mathematics A random sample of 100 diabetics undergoes genotyping and it was found that 23 of them have the gene. Compute the Z test statistic needed for this hypothesis test....
- W World Languages Nina asked her parents for a loan because she wanted to go to Spain with her friends. exclamatory declarative imperative interrogative...

Ответ:
Explanation:
Any change in the equilibrium is studied on the basis of Le-Chatelier's principle.
This principle states that if there is any change in the variables of the reaction, the equilibrium will shift in the direction to minimize the effect.
For the given equation:
Given that reaction is an endothermic reaction.
For the given options:
a)Adding more C
If the concentration of C that is the reactant is increased, so according to the Le-Chatlier's principle, the equilibrium will shift in the direction where decrease of concentration of C takes place. Therefore, the equilibrium will shift in the right direction to wards the formation of hydrogen gas.
b) Adding more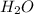
If the concentration of water that is the reactant is increased, so according to the Le-Chatlier's principle, the equilibrium will shift in the direction where decrease of concentration of water takes place. Therefore, the equilibrium will shift in the right direction towards the formation of hydrogen gas.
c) Raising the temperature of the reaction mixture
If the temperature is increased,heat of the equilibrium mixture will also increase so according to the Le-Chatlier's principle , the equilibrium will shift in the direction where decrease in heat that is decrease in temperature occurs.
As, this is an endothermic reaction, forward reaction will decrease the temperature. Hence, the equilibrium will shift in the right direction that is towards the formation of hydrogen gas.
d) Increasing the volume of reaction mixture
If the volume of the container is increased, the pressure will decrease according to Boyle's Law. Now, according to the Le-Chatlier's principle, the equilibrium will shift in the direction where increase in pressure is taking place. As the number of moles of gas molecules is greater at the product side. So, the equilibrium will shift in the right direction that is towards the formation of hydrogen gas.
e) Adding a catalyst to reaction mixture
Role of catalyst is to attain the equilibrium quickly without disturbing the state of equilibrium. Hence, addition of catalyst will not change the equilibrium of the reaction.
f) Adding an inert gas to reaction mixture
Adding inert gas to the mixture at constant volume will not effect the equilibrium. Hence, addition of an inert gas will not change the equilibrium of the reaction.
Ответ:
Step-by-step explanation:
Since there’s 3 feet in one yard, we can multiply the amount of yards by 3 to find out the amount of feet
3 x 2160 = 6480 feet