blymouton13
26.11.2019 •
Chemistry
Consider the following reaction at constant pressure. use the information provided below to determine the value of δs at 473 k. predict whether or not this reaction will be spontaneous at this temperature.
4nh3 (g) + 3o2 (g) → 2n2 (g) + 6h2o (g) δh = –1267 kj
Solved
Show answers
More tips
- P Philosophy Personal attitude towards Confession: how to prepare and undergo the procedure correctly?...
- H Health and Medicine Flu: How to Recognize It by the First Symptoms?...
- F Food and Cooking How to Sober Up Quickly? Important Facts and Tips...
- H Health and Medicine How to Properly Take a Blood Sugar Test?...
- H Health and Medicine Simple and Effective: How to Get Rid of Cracked Heels...
- O Other How to Choose the Best Answer to Your Question on The Grand Question ?...
- L Leisure and Entertainment History of International Women s Day: When Did the Celebration of March 8th Begin?...
- S Style and Beauty Intimate Haircut: The Reasons, Popularity, and Risks...
- A Art and Culture When Will Eurovision 2011 Take Place?...
- S Style and Beauty How to Choose the Perfect Hair Straightener?...
Answers on questions: Chemistry
- E English Explain the significance of the title Two kinds and how its meaning is developed over the course of the story. Use evidence from the text to support your response....
- M Mathematics B. six packages had weights of 2 lb, 5 lb, 7 lb, 5 lb, 9 lb, and 8 lb. what’s the average weight of the packages?...
- P Physics You have an empty milk container and you close the On the container to the particles of air that you trapped inside the container have a mass...
- S Social Studies Favoring the Idea of national. regulation of trade between states would be an example of which political party s stance listed in the chart? 1. The federalist party s belief...

Ответ:
The reaction will be spontaneous
Explanation:
To determine if the reaction will be spontaneous or not at this temperature, we need to calculate the Gibbs's energy using the following formula:
If the Gibbs's energy is negative, the reaction will be spontaneous, but if it's positive it will not.
Calculating the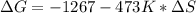 :
:
Now, other factor we need to determine is the sign of the S variation. When talking about gases, the more moles you have in your system the more enthropic it is.
In this reaction you go from 7 moles to 8 moles of gas, so you can say that you are going from one enthropy to another higher than the first one. This results in:![\Delta S0[/tex}Back to this expression: [tex]\Delta G= -1267 - 473 K* \Delta S](/tpl/images/0392/0613/808f0.png)
If the variation of S is positive, the Gibbs's energy will be negative always and the reaction will be spontaneous.
Ответ: