rosieposie27
24.09.2019 •
Chemistry
Deep sea divers use a mixture of helium and oxygen to breathe. assume that a diver is going to a depth of 150 feet where the total pressure is 4.42 atm. the partial pressure of oxygen at this depth is to be maintained at 0.20 atm, the same as at sea level. what must be the percent by volume of oxygen in the gas mixture?
Solved
Show answers
More tips
- S Style and Beauty Lamination of Hair: How it Works and What it is?...
- C Computers and Internet Best Applications for Your iPad: Review of the Best Candidates for Installation...
- S Style and Beauty Intimate Haircut: The Reasons, Popularity, and Risks...
- A Art and Culture When Will Eurovision 2011 Take Place?...
- S Style and Beauty How to Choose the Perfect Hair Straightener?...
- F Family and Home Why Having Pets at Home is Good for Your Health...
- H Health and Medicine How to perform artificial respiration?...
- H Health and Medicine 10 Tips for Avoiding Vitamin Deficiency...
- F Food and Cooking How to Properly Cook Buckwheat?...
- F Food and Cooking How Many Grams Are In a Tablespoon?...
Answers on questions: Chemistry
- C Chemistry Which of the following contains the highest number of electrons? OA) hydroxide ion OB) oxonium ion C) ammonium ion OD) oxide ion O E) all of them contains the same number of...
- C Chemistry A gas sample has a volume of 300.0 L when under a pressure of 3atm. What is the new volume iſ the pressure is increased to 4 atm while the temperature is held constant? 200L...
- C Chemistry A chemistry student needs of acetic acid for an experiment. She has available of a w/w solution of acetic acid in benzene. Calculate the mass of solution the student should...
- C Chemistry In an experiment to compare two pain relievers, seven subjects took one of the two pain relievers (randomly selected) for two weeks, then switched to the other for another...
- E English What is the first step in the six-step process for preparing a speech? a. selecting an appropriate topic b. gathering information for your speech c. practicing your speech...
- P Physics How much work does the force of gravity do in pulling a 10 kg box down a 30º inclined plane of length 8.0 m? note that sin 30 = cos 60 = 0.500 and cos 30 = sin 60 = 0.866....
- M Mathematics What s the total value of the digit 6 in 836...
- P Physics How can a driver best prepare to enter sharp curves on a road way...
- H History What did all the farmers of the constitution agree to before the convention? ! ?...
- H Health Are a group of cells called an organ...

Ответ:
4.525% is the percentage by volume of oxygen in the gas mixture.
Explanation:
Total pressure of the mixture = p = 4.42 atm
Partial pressure of the oxygen =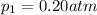
Partial pressure of the helium =
According Avogadro law:
Volume occupied by oxygen gas =
Total moles of gases = n = 1 mol
Total Volume of the gases = V
Percent by volume of oxygen in the gas mixture:
Ответ:
I hope this helps