dinosaur10
09.08.2019 •
Chemistry
Exactly 1.0 mol n2o4 is placed in an empty 1.0-l container and is allowed to reach equilibrium described by the equation n2o4(g) ↔ 2no2(g) if at equilibrium the n2o4 is 37% dissociated, what is the value of the equilibrium constant, kc, for the reaction under these conditions?
Solved
Show answers
More tips
- B Business and Finance How to Open an Online Store? A Detailed Guide for Beginners...
- W Work and Career How to Write a Resume That Catches the Employer s Attention?...
- C Computers and Internet Е-head: How it Simplifies Life for Users?...
- F Family and Home How to Choose the Best Diapers for Your Baby?...
- F Family and Home Parquet or laminate, which is better?...
- L Leisure and Entertainment How to Properly Wind Fishing Line onto a Reel?...
- L Leisure and Entertainment How to Make a Paper Boat in Simple Steps...
- T Travel and tourism Maldives Adventures: What is the Best Season to Visit the Luxurious Beaches?...
- H Health and Medicine Kinesiology: What is it and How Does it Work?...
- O Other How to Choose the Best Answer to Your Question on The Grand Question ?...
Answers on questions: Chemistry
- C Chemistry Oxygen-19 has a half-life of 29.4 seconds. How long would it take for a 1200g sample to decay to 20g? Round to 2 decimals.j...
- C Chemistry How did fuel efficiency change between 1965-2010...
- C Chemistry Proteins, carbohydrates, and DNA are similar in that they are A. all organic compounds B. parts of your hereditary information C. all made of amino acids D. all made...
- C Chemistry Substance c was heated at first gently and thenmore strongly in abunsen flame.A yellow residue which cooled to awhite solid was formed. A gas thatturns lime water...
- C Chemistry Show your neat work including correct sig figs and units. Use a stylus or zoom in to help with neat writing. * Clear Gerardo needs 2.00 moles of Magnesium Fluoride....
- C Chemistry Which is the best definition of a CHARACTERISTC physical property?...
- G Geography First glance, the governments of the United States and Canada may appear similar. It is true that both nations share many of the same democratic principles. They...
- M Mathematics 3. Noel Magee purchased a micro-cut shredder at a sale price of $179.99. The regular selling price is $199.99. He also purchased a photo-smart printer at 25% off...
- S Spanish Why Selena become so famous? by the way the music girlplease write 3 or more sentences...
- C Chemistry Steps in order to calculate the average speed between two points of a distance-time graph...

Ответ:
Answer : The equilibrium constant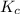 for the reaction is, 0.869
for the reaction is, 0.869
Explanation :
First we have to calculate the concentration of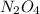 .
.
The balanced equilibrium reaction is,
Initial conc. C 0
At eqm. conc.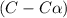
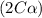
As we are given,
The percent of dissociation =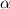 = 37 % = 0.37
= 37 % = 0.37
Now we have to calculate the equilibrium constant for the reaction.
The expression of equilibrium constant for the reaction will be :
Now put all the values in this expression, we get :
Therefore, the equilibrium constant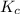 for the reaction is, 0.869
for the reaction is, 0.869
Ответ:
always repeats the same unit
made of smaller molecules
often made from repeating units