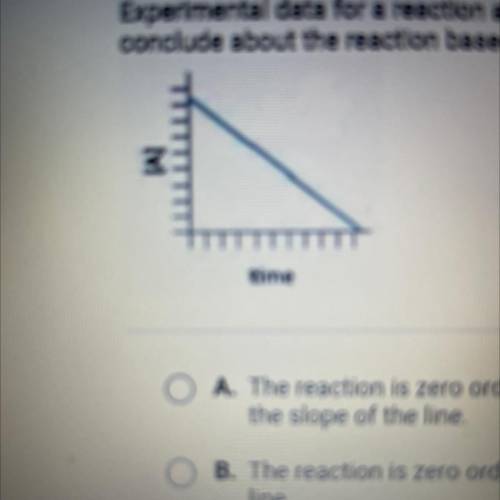
Experimental data for a reaction are collected and graphed. What can you
conclude about the reaction based on the graph?
A. The reaction is zero order, and the rate constant is the negative of
the slope of the line
B. The reaction is zero order, and the rate constant is the slope of the
line
C. The reaction is first order, and the rate constant is the slope of the
line,
D. The reaction is first order, and the rate constant is the negative eg
Solved
Show answers
More tips
- C Computers and Internet Porn Banner: What It Is and How to Get Rid Of It?...
- C Computers and Internet Отправляем смс через интернет: легко и просто...
- L Leisure and Entertainment The Best Film of 2010: A Look Back at the Academy Awards...
- H Health and Medicine Simple and Effective: How to Get Rid of Cracked Heels...
- O Other How to Choose the Best Answer to Your Question on The Grand Question ?...
- L Leisure and Entertainment History of International Women s Day: When Did the Celebration of March 8th Begin?...
- S Style and Beauty Intimate Haircut: The Reasons, Popularity, and Risks...
- A Art and Culture When Will Eurovision 2011 Take Place?...
- S Style and Beauty How to Choose the Perfect Hair Straightener?...
- F Family and Home Why Having Pets at Home is Good for Your Health...
Answers on questions: Chemistry
- C Chemistry What is the relationship between the circulatory and respiratory systems? A. They work together to store the waste that blood collects from the body.B. The circulatory system...
- C Chemistry Please help me I need these answers...
- C Chemistry What are substances that consist of combinations of 2 or more pure substances, or particles that can be in any form of solids, liquids, and/or gases?...
- C Chemistry What is meant by the average atomic mass of an element?...
- C Chemistry Use the specific heat of water to determine how much heat is required to raise the temperature of 50.0g of water from 35oc to 55oc....
- C Chemistry The volume of a sphere is given by v= (4/3) pi r cubed, where r is the radius. compute the volume of a sphere with a radius of 117pm. state your answer in units of cubed....
- C Chemistry What is the smallest component or the most basic building block of any element ? a. an atom, b.a compound c.gas d.element...
- E English There s nothing you can do that can t be done meaning...
- E English An author may describe a character by telling what he does, what he says, how he looks, or how others react to him....
- B Business Describes the degree of interdependence among modules....

Ответ:
1.33 Å
Explanation:
Given that the edge length , a of the KCl which forms the FCC lattice = 6.28 Å
Also,
For the FCC lattice in which the anion-cation contact along the cell edge , the ratio of the radius of the cation to that of anion is 0.731.
Thus,
Also, the sum of the radius of the cation and the anion in FCC is equal to half of the edge length.
Thus,
Given that:
To find,
Using 1 and 2 , we get:
Size of the potassium ion = 1.33 Å