kiki197701
01.04.2020 •
Chemistry
Given the following standard reduction potentials at 25°C:
Half reaction
Ag (aq) + e → Ag (S)
Cu(ag) + 2e → Cu (S)
Ni?' (ag) + 2e → Ni (s)
Cr+ (aq) + 3e → Cr(s)
Reduction Potential (V)
+ 0.80
+0.34
- 0.28
-0.74
1. Combine these reduction half-reactions into a redox reaction (hint-one will be a
reduction, the other will be an oxidation) such that the Ecell is the smallest positive value
possible.
Balanced redox reaction:
Eceh
mV
2. What is 'n' from the reaction above? n=
moles electrons
3. What is delta G and is this reaction spontaneous or not? delta G=
4. What is K?K=
5. Is the reaction product-favored or reactant favored?
Solved
Show answers
More tips
- F Family and Home How to Sew Curtain Tapes: Best Tips from Professionals...
- A Animals and plants How to Grow Lime from a Seed: Simple Tips and Interesting Facts...
- C Computers and Internet How to Create a Folder on Your iPhone?...
- G Goods and services How to sew a ribbon: Tips for beginners...
- F Food and Cooking How to Make Mayonnaise at Home? Secrets of Homemade Mayonnaise...
- C Computers and Internet Which Phone is Best for Internet Surfing?...
- F Food and Cooking Everything You Need to Know About Pasta...
- C Computers and Internet How to Choose a Monitor?...
- H Horoscopes, Magic, Divination Where Did Tarot Cards Come From?...
Answers on questions: Chemistry
- E English 2 What character trait does Macbeth reveal as he meets his fate? Explain your positioning and...
- H History An african american singer and songwriter named otis redding wrote a song called respect in 1965 which included the line all i m askin is for a little respect. why would...
- M Mathematics Find the measure of each angle: PLS HELP...
- C Chemistry What is the source of the litmus paper explain its use...

Ответ:
3.36 L of ammonia gas
Explanation:
The balanced chemical reaction is:
According to stoichiometry :
3 moles of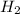 produce = 2 moles of
produce = 2 moles of 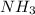
Thus 0.75 moles of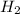 will producee=
will producee=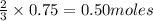 of
of 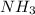
But as percent yield is 30 %, amount of ammonia produced =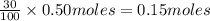
According to ideal gas equation:
P = pressure = 1 atm
V = Volume = ?
n = number of moles = 0.15
R = gas constant =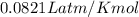
T =temperature =
Thus 3.36 L of ammonia gas is obtained by reacting 0.75 moles of hydrogen with excess nitrogen.