alyssacruz999
12.08.2020 •
Chemistry
Human lungs have evolved to breathe oxygen at a pressure as that in the atmosphere, 0.21 atm. If a particular heliox mixture to be carried by a scuba diver is at a pressure of 7.00 atm, what should be the partial pressure due to helium in order to maintain the pressure due to oxygen at 0.21 atm?
Solved
Show answers
More tips
- C Computers and Internet How to Download Videos from YouTube? Simple Steps to Download Any Content...
- S Style and Beauty Tricks and Tips: How to Get Rid of Freckles...
- H Health and Medicine How to perform artificial respiration?...
- C Computers and Internet How to Get Rid of Windows Genuine Check?...
- F Food and Cooking The Disease That Haunted Abraham Lincoln...
- S Style and Beauty How to Make Your Lips Fuller? Ideas and Tips for Beautiful Lips...
- S Style and Beauty How are artificial nails removed?...
- F Family and Home How to Sew Curtain Tapes: Best Tips from Professionals...
- H Horoscopes, Magic, Divination How to Cast a Love Spell on a Guy? Guide for Guys...
- F Family and Home How to Properly Use a Water Level?...
Answers on questions: Chemistry
- C Chemistry What is the subscript of Carbon in Isovaleric acid C5H10O2...
- C Chemistry How many grams of iron, Fe, are produced with 25.0 grams of magnesium, Mg?...
- C Chemistry Using your understanding ofmeiosis, explain why human offspring may have similar traits but are not identical...
- C Chemistry Ultraviolet radiation and radiation of shorter wavelengths can damage biological molecules because they carry enough energy to break bonds within the molecules....
- C Chemistry Fifty tickets,numbered consecutively 1 to fifty are placed in a box. what is the probability that in 4 separate drawing the following solutions occur? a) odd numbers,if...
- C Chemistry On a cold winter day, the temperature is -23.7 oF. What is that temperature in degrees Celsius? Make sure to include the correct unit symbol with the answer....
- C Chemistry Calculate the formula mass of your mineral in amu to 2 decimal places for the mineral Mendozite ITS DUE TONIGHT...
- C Chemistry What would be the products of the complete hydrolysis of val-ser-his?...
- C Chemistry What is the [oh-]if the ph of a solution 10.62...
- C Chemistry Asolution made by dissolving 9.81 g of a nonvolatile nonelectrolyte in 90.0 g of water boiled at 100.37degree c at 760 mm hg. what is the approximate molecular weight...

Ответ:
6.79 atm
Explanation:
Applying Dalton's law of partial pressure:
From the illustration,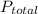 = 7.00 atm and
= 7.00 atm and 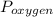 = 0.21 atm. Hence, the partial pressure due to helium is calculated such that:
= 0.21 atm. Hence, the partial pressure due to helium is calculated such that:
= 7.00 - 0.21
= 6.79 atm
Therefore, the partial pressure due to helium in order to maintain the pressure due to oxygen at 0.21 atm would be 6.79 atm.
Ответ:
Sample Response: If the data is collected in a biased manner, the graph could be skewed. Also, if the number of observations is too small, the graph can be skewed. To ensure the data is not skewed, collect a large representative unbiased sample