michaellagann2020
28.06.2019 •
Chemistry
In the covalent bond formation process orbitals from each atom overlap and electrons are shared between each atom. you can visualize the 1s atomic orbital of one hydrogen atom overlapping with the 1s orbital of the other hydrogen atom to form an hâh covalent bond. each hydrogen atom in the h2 molecule has the electron configuration analogous to that of the he atom. similarly, when 2p orbitals of two fluorine atoms overlap they share one electron each. the total number of electrons in each f atom is nine. each f atom shares one electron with the other f atom, and thus the total number of electrons in each f atom in the f2 molecule is 10. therefore, each f atom in the f2 molecule has the electron configuration analogous to that of ne. the atomic orbitals of two iodine atoms combine to form the diatomic i2 molecule. use the periodic table to determine the atomic orbitals that overlap to form the i2 molecule and the symbol of the noble gas that has the same electron configuration as the electron configuration of each bonded iodine atom. for example, the 2p atomic orbitals of fluorine atoms overlap to form the f2 molecule. the noble gas that has the same electron configuration as that of each bonded fluorine atom is ne. to enter the atomic orbitals that overlap and the corresponding noble gas, you would enter 2p,ne. enter the symbol for the orbitals that overlap and the chemical symbol of the noble gas separated by a comma. for example, for h2 enter 1s, he.
Solved
Show answers
More tips
- P Philosophy Agnosticism: Opinion or Belief?...
- S Style and Beauty How to choose the best mascara for your eyelashes...
- F Food and Cooking Discover Delicious Recipes You Can Make with Ground Meat...
- C Computers and Internet Google Search Tips and Tricks: Everything You Need to Know...
- S Science and Technology Why is there no gravity on other planets?...
- L Leisure and Entertainment How to Properly Wind Fishing Line onto a Reel?...
- L Leisure and Entertainment How to Make a Paper Boat in Simple Steps...
- T Travel and tourism Maldives Adventures: What is the Best Season to Visit the Luxurious Beaches?...
- H Health and Medicine Kinesiology: What is it and How Does it Work?...
- O Other How to Choose the Best Answer to Your Question on The Grand Question ?...
Answers on questions: Chemistry
- M Mathematics Which of the following statements best justifies why \overleftrightarrow{CG} CG C, G, with, \overleftrightarrow, on top is parallel to \overleftrightarrow{AB} AB A, B, with, \overleftrightarrow,...
- G Geography How is a meander of a river formed...
- M Mathematics (6.6 × 10⁰) + (7.8 × 10²)...
- M Mathematics A membership at Gisele s Gym costs $145 to join and $3 for each visit. A membership at Freddie s Fitness costs $75 to join and $5 for each visit. At how many visits will both cost...
- M Mathematics What is the cost of constructing a fence 6 ft., 6 in. high around a lot measuring 90 feet by 175 feet, if the cost of erecting the fence is $1.25 per linear foot, and the cost of...

Ответ:
The atomic orbitals which combine to form molecule is 5p-orbitals and the noble gas is Xenon (Xe).
molecule is 5p-orbitals and the noble gas is Xenon (Xe).
Explanation: Iodine is the 53rd element of the periodic table. It lies in Group 17 of the periodic table.
Valence shell electronic configuration of Iodine =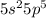
5p orbitals of two iodine atoms overlap to share one electron each. The total number of electrons that each iodine atom contain are 53. Each iodine atom shares one electron with the other iodine atom, so that each molecule of contains 54 electrons. Hence, the electronic configuration of noble gas Xenon is same as the each iodine atom in
contains 54 electrons. Hence, the electronic configuration of noble gas Xenon is same as the each iodine atom in  molecule.
molecule.
Therefore, 5p orbital overlap to form molecule and the noble gas having same number of electrons as each iodine atom in
molecule and the noble gas having same number of electrons as each iodine atom in  molecule is Xenon. the symbol for this gas is 'Xe'.
molecule is Xenon. the symbol for this gas is 'Xe'.
Ответ:
YEEHAWW MA FAVORITE OL' PETS ARE LIL' PIGGIES AND SOME DONKEYS TOO HE HE HE. PIGGIES RULE. HE HE HE
OL' LIL' COUNTRY SHALL I THEEE
YYY