teescub9738
10.09.2019 •
Chemistry
In the following experiment, a coffee-cup calorimeter containing 100 ml of h2o is used. the initial temperature of the calorimeter is 23.0 ∘c. if 3.00 g of cacl2 is added to the calorimeter, what will be the final temperature of the solution in the calorimeter? the heat of solution δhsoln of cacl2 is −82.8 kj/mol. assume that the specific heat of the solution form
Solved
Show answers
More tips
- P Philosophy Agnosticism: Opinion or Belief?...
- S Style and Beauty How to choose the best mascara for your eyelashes...
- F Food and Cooking Discover Delicious Recipes You Can Make with Ground Meat...
- C Computers and Internet Google Search Tips and Tricks: Everything You Need to Know...
- S Science and Technology Why is there no gravity on other planets?...
- L Leisure and Entertainment How to Properly Wind Fishing Line onto a Reel?...
- L Leisure and Entertainment How to Make a Paper Boat in Simple Steps...
- T Travel and tourism Maldives Adventures: What is the Best Season to Visit the Luxurious Beaches?...
- H Health and Medicine Kinesiology: What is it and How Does it Work?...
- O Other How to Choose the Best Answer to Your Question on The Grand Question ?...
Answers on questions: Chemistry
- C Chemistry The further apart objects are, the the gravity between them....
- M Mathematics Need help asap plss i’ll give brainliest !...
- H History Best Tv advertisement that convinces people to buy TV time period 1950 1 paragraph will make as brain list...
- M Mathematics Find the missing terms in the sequence, 21, 18, 15, 12, To find the next term, add___ This is a(n) (arithmetic or geometric) sequence...

Ответ:
28.3 °C
Explanation:
We assume that the specific heat of the solution is equal to the specific heat of water (4.184 Jg⁻¹ °C⁻¹).
First, we find the heat released by the dissolution of CaCl₂. The grams will be converted to moles using the molar mass (110.986 g/mol), then multiplied by the molar heat of solution:
(3.00 g)(mol/110.986 g)(-82.8 kJ/mol) = -2.23812 kJ
The negative sign indicates that heat is released. Extra significant figures are included to avoid round-off errors.
The amount of heat released by the CaCl₂ dissolution is equal to the heat absorbed by the water. The equation is rearranged to solve for Δt, the temperature change of the water.
Q = mcΔt ⇒ Δt = Q/(mc)
Δt = (2.23812 kJ)(1000 J/kJ) / (100 mL)(1 g/mL)(4.184 Jg⁻¹ °C⁻¹) = 5.3 °C
We can then calculated the final temperature t₂ of the solution:
Δt = t₂ - t₁
t₂ = Δt + t₁ = 5.3 °C + 23.0 °C = 28.3 °C
Ответ:
When 3.00 g of CaCl₂ is added to a calorimeter containing 100 mL of water at 23.0°C, the final temperature is 28.2 °C.
First, we will convert 3.00 g of CaCl₂ to moles using its molar mass (110.98 g/mol).
The heat of solution (ΔHsoln) of CaCl₂ is −82.8 kJ/mol. The heat released by the solution of 0.0270 moles is:
According to the law of conservation of energy, the sum of the heat released by the solution (Qs) and the heat absorbed by the calorimeter (Qc) is zero.
Assuming the density of water is 1 g/mL, we have 100 mL (100 g) of water and 3.00 g of CaCl₂. The mass of the solution (m) is:
Finally, we can calculate the final temperature of the system using the following expression.
where,
c: specific heat of the solution (same as water 4.18 J/g.°C)
T₁ and T₂: initial and final temperature
When 3.00 g of CaCl₂ is added to a calorimeter containing 100 mL of water at 23.0°C, the final temperature is 28.2 °C.
Learn more: link
Ответ:
0.11 moles of the gas are present in the sample of dry gas.
Explanation:
Data given:
mass of the gas = 2.1025 grams
volume of the gas = 2.850 litres
temperature = 22 degrees (273.15+22) = 295.15 K
Pressure = 740 mm Hg or 0.973 atm
moles of the gas =?
R = 0.08206 atmL/Mole K
From the ideal gas law the number of moles can be calculated in the sample of dry gas. Number of moles will be determined by the pressure exerted, volume and temperature of the gas.
The formula:
PV = nRT
n =
putting the values in the above equation:
n =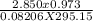
= 0.11 moles
0.11 moles of the dry gas is present in the sample given.