SKYBLUE1015
07.11.2019 •
Chemistry
Initially 2.0 moles of n2(g) and 4.0 moles of h2(g) were added to a 1.0-liter container and the following reaction then occurred: 3h2(g) + n2(g) 2nh3(g) the equilibrium concentration of nh3(g) = 0.55 moles/liter at 700.°c. what is the value for k at 700.°c for the formation of ammonia?
Solved
Show answers
More tips
- S Style and Beauty Why is Sugaring Better than Waxing for Hair Removal?...
- W Work and Career Where can you learn to be a flight attendant?...
- G Goods and services How to Properly Calculate the Power of Your Air Conditioner?...
- F Food and Cooking Effective Methods to Organize Videos in your iPad According to Content...
- F Family and Home Parquet or laminate, which is better?...
- L Leisure and Entertainment How to Properly Wind Fishing Line onto a Reel?...
- L Leisure and Entertainment How to Make a Paper Boat in Simple Steps...
- T Travel and tourism Maldives Adventures: What is the Best Season to Visit the Luxurious Beaches?...
- H Health and Medicine Kinesiology: What is it and How Does it Work?...
- O Other How to Choose the Best Answer to Your Question on The Grand Question ?...
Answers on questions: Chemistry
- C Chemistry A20.0 ounce bottle of mountain dew contains 77.0 g of sugar. how many calories would a person consume in a 365 day year if they drank one 20.0 ounce bottle of mountain...
- C Chemistry The freezing point of 52.71 g of a pure solvent is measured to be 41.99 ºc. when 2.14 g of an unknown solute (assume the van t hoff factor = 1.) is added to the solvent...
- C Chemistry A. identify the limiting reagent in the following system: lithium oxide is used aboard the space shuttle to remove water from the air supply according to the equation:...
- C Chemistry 12 ounce can of a soft drink such as coke classic contains 39.0 g of sugar. 1 fluid ounce classic? 29.57 ml. what is the mass/volume percent of sugar in coke...
- C Chemistry Astudent answered 119 questions correctly on a test and earned a score o 85%. how many questions were on the test?...
- C Chemistry Draw the structure of the amino acid that predominates: a. asparagine at ph=7.4 b. tyrosine at ph=9.5 c. proline at ph=6.5...
- C Chemistry To meet the grand challenge of climate change and reduce the rate of warming of the atmosphere we must begin to remove major sources of greenhouse gases from the...
- C Chemistry Astudent determined the mass of a 100 ml volumetric flask and then prepared a 4% sugar solution by transferring 4.00 g of sugar to the flask and diluting to the mark...
- C Chemistry Submit your answer for the remaining reagent in tutorial assignment #1 question 9 here, including units. note: use e for scientific notation formatting (ie, write...
- C Chemistry Coke classic contains 35 mg of sodium per 8 ounces. a typical soft drink can holds 12 ounces. how many mg of sodium are contained in 12 ounces of coke classic?...

Ответ:
Answer : The value of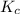 for the reaction is,
for the reaction is, 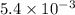
Solution : Given,
Moles of = 2.0 mol
= 2.0 mol
Moles of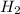 = 4.0 mol
= 4.0 mol
Volume of solution = 1.0 L
Concentration of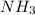 at equilibrium = 0.55 mol/L = 0.55 M
at equilibrium = 0.55 mol/L = 0.55 M
First we have to calculate the concentration of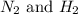 .
.
Now we have to calculate the value of equilibrium constant.
The given equilibrium reaction is,
Initially conc. 2.0 4.0 0
At eqm. (2.0-x) (4.0-3x) 2x
The expression of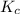 will be,
will be,
The concentration of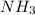 at equilibrium = 0.55 M = 2x
at equilibrium = 0.55 M = 2x
So,
2x = 0.55
x = 0.27 M
Now put the value of 'x' in the above equation 1, we get:
Therefore, the value of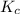 for the reaction is,
for the reaction is, 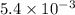
Ответ:
.72 (mol/L)/s
Explanation:
A P E X