wbrandi118
22.03.2021 •
Chemistry
QUESTION 6 Consider the following reaction between the diatomic and monatomic forms of iodine: I2 (g) <-> 2I (g) When 0.095 M I2 is initially placed in a previously empty container and sealed, the system slowly reaches equilibrium. When equilibrium is reached, it is found that there is an equilibrium concentration of 0.0055 M of the monatomic form of iodine. Calculate the (unitless) equilibrium constant Kc. Round your answer to two sig figs, and express it in scientific notation.
Solved
Show answers
More tips
- F Food and Cooking The Health Benefits of Flaxseed oil...
- S Style and Beauty Why is Sugaring Better than Waxing for Hair Removal?...
- W Work and Career Where can you learn to be a flight attendant?...
- G Goods and services How to Properly Calculate the Power of Your Air Conditioner?...
- F Food and Cooking Effective Methods to Organize Videos in your iPad According to Content...
- F Family and Home Parquet or laminate, which is better?...
- L Leisure and Entertainment How to Properly Wind Fishing Line onto a Reel?...
- L Leisure and Entertainment How to Make a Paper Boat in Simple Steps...
- T Travel and tourism Maldives Adventures: What is the Best Season to Visit the Luxurious Beaches?...
- H Health and Medicine Kinesiology: What is it and How Does it Work?...
Answers on questions: Chemistry
- C Chemistry Does Arsenic have a high or low ionization energy in comparison to Carbon? Explain why using the concepts of the structure of the atom...
- C Chemistry Please help ASAP please. Correct answer please...
- C Chemistry Carefully add HNO into the flask until the phenolphthalein begins to lose its color. Stop adding HNO3 when the color change is permanent. A. How much (HNO3) was required to cause...
- C Chemistry In which part of a product formed versus time graph has the reaction reached completion? A. the steepest part B. the vertical part C. the horizontal part...
- C Chemistry What are some examples of a diprotic binary acid...
- C Chemistry What volume, in mL, of a 0.200 M H3PO4 solution is needed to react with 120. mL of 0.100 M NaOH solution? H3PO4 + 3NaOH à Na3PO4 + 3H2O...
- C Chemistry What is the empirical formula for a compound that has 57.5% Na, 40.0% 0, and 2.5% H? a Naz(OH)4 b Na(OH)2 С Na(OH) d Naz(OH)...
- C Chemistry 4 grams of hydrogen and 32 grams of oxygen will combine to form: A: 36 grams B: 28 grams of hydroxide C: 32 grams of Oxygen D: 36 grams of deuterium...
- H Health En qué división se encuentra Argentina según el criterio físico natural...
- H History It can be your opinion i dont mind, this is due basically right now so if you could please help :))...

Ответ:
The equilibrium constant is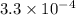
Explanation:
Initial concentration of = 0.095 M
= 0.095 M
The given balanced equilibrium reaction is,
Initial conc. 0.095 M 0 M
At eqm. conc. (0.095-x) M (2x) M
Given : 2x = 0.0055
x = 0.00275
The expression for equilibrium constant for this reaction will be,
Now put all the given values in this expression, we get :
Thus the equilibrium constant is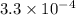
Ответ:
the renaissance was a historical movement that started in italy but spread through europe, that symbolized the end of the middle age and the beginning of the modern age.
this period in time was characterized by innovation, creativity, imagination and restoration of principles and moral values. in this time, europe classic past history was revisited and put up for debate. it was a social, political, artistic and literary movement that began as an effort to emulate the societies of classic ancient greece and rome.