Miguel1310
02.08.2021 •
Chemistry
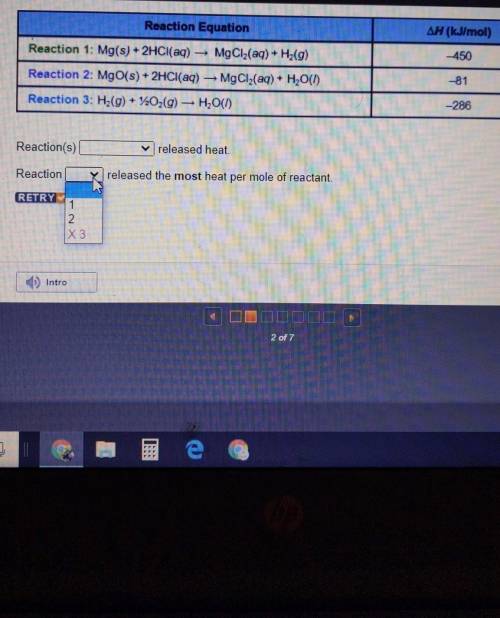
Reaction Equation ∆H (kJ/mol) -450 Reaction 1: Mg(s) + 2HCl(aq) — MgCl2(aq) + Hz(9) Reaction 2: MgO(s) + 2HCl(aq) — MgCl2(aq) + H2O(1) Reaction 3: H2(g) + 220,(9) — H2O(1) -81 -286
Reaction(s)_______released heat. W
Reaction________released the most heat per mole of reactant
Solved
Show answers
More tips
- H Health and Medicine How Many Ribs Do Humans Have?...
- H Health and Medicine Simple and Effective: How to Get Rid of Cracked Heels...
- H Health and Medicine Relieving Swelling in Legs: Causes and Ways to Alleviate the Symptom...
- W Work and Career Мерчендайзинг – все, что нужно знать...
- O Other Everything You Need to Know About Kudyabliks...
- F Food and Cooking How to cook crayfish? Everything you need to know...
- F Food and Cooking Homemade kvass: recipe and brewing process...
- H Health and Medicine How to Choose the Right Tanning Cream?...
- S Style and Beauty Secrets of Tying a Pareo: 5 Ways...
- S Sport Running: How to Do It Right?...

Ответ: