Suppose the first equation is reversed and multiplied by 1/6, the second and third equations are divided by 2, and the three adjusted equations are added. What is the net reaction?3 A + 6 B → 3 D, ΔH = -402 kJ/molE + 2 F → A, ΔH = -108.3 kJ/molC → E + 3 D, ΔH = +65.5 kJ/mol
Solved
Show answers
More tips
- F Family and Home How to Choose a Name for Your Baby?...
- B Business and Finance How to Open an Online Store? A Detailed Guide for Beginners...
- W Work and Career How to Write a Resume That Catches the Employer s Attention?...
- C Computers and Internet Е-head: How it Simplifies Life for Users?...
- F Family and Home How to Choose the Best Diapers for Your Baby?...
- F Family and Home Parquet or laminate, which is better?...
- L Leisure and Entertainment How to Properly Wind Fishing Line onto a Reel?...
- L Leisure and Entertainment How to Make a Paper Boat in Simple Steps...
- T Travel and tourism Maldives Adventures: What is the Best Season to Visit the Luxurious Beaches?...
- H Health and Medicine Kinesiology: What is it and How Does it Work?...
Answers on questions: Chemistry
- C Chemistry When the atoms of two substances joined to make a new substance the process is called?...
- C Chemistry 4. in an atomic model, what particles are found in the area surrounding the nucleus?...
- C Chemistry Please help :,) A 45.00-g sample of silver nitrate is mixed with 55.00 g of hydrochloric acid to form a white precipitate of silver chloride. After the solution is filtered and...
- C Chemistry A. 35 b.65 c.81 d.180...
- C Chemistry in an endothermic reaction, the have more energy than the .A. surroundings, productsB. reactants, productsC. products, reactants D. reactants, surroundings...
- H Health What kind of job did Einstein get in in 1902 ? what was he supposed to do...
- E English The Bharatnatyam dancer practised for several hours to perform well in her Arangetram (first performance). The dancer can be described as being . A hardworking B good C creative...
- S Social Studies What is the Illuminati?...
- M Mathematics 2 Points What is the vertex of the graph of this equation y=-4x2 - 16x - 12? O A. (-2,-4) O B. (2, 4) O C. (2, -4) D. (-2,4) Help need this solved fast...
- M Mathematics A toy store has 10 stores all about the same size in a city the graph shows sales for one of the stores last month. Which statement is best supported by the information in the...

Ответ:
Explanation:
From the question; suppose the first equation is reversed and multiplied by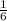 ; we have:
; we have:
3D ------> 3A + 6B ; ΔH = + 402 kJ/mol
The second and third equations are divided by 2; so are going to have the following:
E + 2 F -------> A ;ΔH = -108.3 kJ/mol
C -------> E + 3 D ;ΔH = +65.5 kJ/mol
Adding these three adjusted equations; we have:
∴ The net reaction =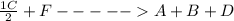 ;ΔH = + 45.6 kJ/mol
;ΔH = + 45.6 kJ/mol
Ответ:
Strong Surface Tension, Covalent Bonds, Denser as a Liquid, Polar.
Explanation:
Water is a polar substance with strong surface tension. It is denser as a liquid than as a solid. A water molecule consists of a hydrogen atom covalently bonded to two oxygen atoms.